Taoufiq Benali1* , Houda Chtibi1
, Houda Chtibi1 , Abdelhakim Bouyahya2
, Abdelhakim Bouyahya2 , Abdelmajid Khabbach3
, Abdelmajid Khabbach3 and Khalil Hammani1
and Khalil Hammani1
1Laboratory of Natural Resources and Environment, Polydisciplinary Faculty of Taza, Sidi Mohamed Ben Abdellah University of Fez B.P.: 1223, Taza-Gare. Taza, Morocco
2Laboratory of Human Pathologies Biology, Department of Biology, Faculty of Sciences, and Genomic Center of Human Pathologies, Faculty of Medicine and Pharmacy, Mohammed V University in Rabat, Morocco
3Laboratory of Materials, Natural Substances, Environment and Modeling (LMSNEM), Polydisciplinary Faculty of Taza, Sidi Mohamed Ben Abdellah University of Fez B.P.: 1223, Taza-Gare. Taza, Morocco
Corresponding Author E-mail : benali.taoufiq@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1924
Abstract
The present study reports the phenol and flavonoid contents of Cistus ladaniferus and Mentha suaveolens from Northern Morocco and the evaluation of their in vitro antioxidant and antimicrobial activities against gram positive, gram negative bacteria, and yeast. The results showed that the highest phenol and flavonoid content found in the aqueous extract of Cistus ladaniferus. However, the methanol extract of Mentha suaveolens contains the high phenol and flavonoid contents. A significant anti-DPPH activity was obtained with aqueous extract of Mentha suaveolens and Cistus ladaniferus with IC50 values of 8.78 ± 0.02μg/mL and 2.93± 0.02μg/mL, respectively. An important anticandidal effect of MSEO as observed against Candida albicans. The most sensitive bacteria to all extracts were B. subtilis, P. mirabils and S. aureus. Our findings indicate that the natural compounds of Cistus ladaniferus and Mentha suaveolens with an antimicrobial activity could be a good target for the antioxidants and antimicrobial industry. However, further investigations regarding the isolation of phenolic bioactive compounds and the evaluation of their antioxidant and antimicrobial properties are required.
Keywords
Antioxidant Activity; Antimicrobial Activity; Cistus Ladaniferus; Mentha Suaveolens; Phenol Content
Download this article as:| Copy the following to cite this article: Benali T, Chtibi H, Bouyahya A, Khabbach A, Hammani K. Detection of Antioxidant and Antimicrobial Activities in Phenol Components and Essential oils of Cistus ladaniferus and Mentha suaveolens extracts. Biomed Pharmacol J 2020;13(2). |
| Copy the following to cite this URL: Benali T, Chtibi H, Bouyahya A, Khabbach A, Hammani K. Detection of Antioxidant and Antimicrobial Activities in Phenol Components and Essential oils of Cistus ladaniferus and Mentha suaveolens extracts. Biomed Pharmacol J 2020;13(2). Available from: https://bit.ly/30WURzL |
Introduction
Since antiquity, plants form an important source of metabolite secondary to develop remedies to treat various diseases.1 Recently, several scientific researchers have demonstrated that natural plant extracts possess biological properties such as antimicrobial, antioxidant, anti-diabetic, anticancer, and anti-inflammatory activities.2-7
Cistus Ladaniferus growing naturally in the Mediterranean region, namely in Morocco “Touzalt; touzaṭch” frequently used in traditional medicines to treat many pathology such as stomachic problems and low libido case.8, 9 Several studies of the chemical composition of Cistus Ladaniferus essential oil (CLEO) indicate that its main compounds were viridiflorol, p-cymene, camphene, bornyl acetate, pinocarveol, α-pinene, and ledol.10 Another work identified some flavonoids in the leaf of C. Ladaniferus.11 In addition, a high content of viridiflorol, blumenol, limonene and spathulenol are detected in C. Ladaniferus methanol and ethanol extracts.12 The biological effects of Cistus Ladaniferus as antibacterial, antioxidant, antifungal, and cytotoxic effects of C. Ladaniferus antifungal, are documented by several scientific works.13-17
Mentha Suaveolens (Lamiaceae family) locally known as “m’chichro” is traditionally used to treat cold, digestive system ailment, hemorrhoid, fever, intestinal swelling, flu against chill, and as carminative and conception and fertility booster.8,9,18,19 The genus Mentha is largely distributed in temperate regions of the world.20 Mentha Suaveolens was characterized by free flavonoid.21 Mentha Suaveolens essential oils (MSEO) from different regions were characterized by high levels of menthone, piperitone oxide, pulegone, isomenthone, and piperitenone oxide.22 The oils of many Mentha species exhibit an important biological proprieties.23- 27
This study aims to determine the phenol and flavonoid contents of C. Ladaniferus and M. Suaveolens extracts and to evaluate the antioxidant and antimicrobial activity of these extracts, CLEO and MSEO.
Materials and Methods
Plant Material
Cistus Ladaniferus and M. Suaveolens were collected from Taza region (006° 28, 382′ E 004° 49,405’N and 34°10.510′ N, 004°01.190′ W, respectively) in 2016. The plants were identified in the laboratory of Natural Resources and Environment, Polidisciplinary Faculty of Taza, University Sidi Mohamed Ben Abdellah-Fez. The voucher specimens of plants were deposited FPT-LRNE-31 and FPT-LRNE-11, respectively. The collected material was dried in the laboratory at 25 °C under shade.
Isolation of the Essential Oils
The extraction of CLEO and MSEO were carried out using hydro-distillation method. Indeed, the dried aerial parts (100g) of C. Ladaniferus were and Mentha Suaveolens extracted in a Clevenger-type apparatus for 4 h. Then, CLEO and MSEO were stored at 4°C until use 3.
Solvent Extracts
Dried powder (10g/100 mL) of aerial part of the plant was macerated with ethanol, methanol, and water at ambient temperatures. The extracts were filtered and concentrated using a rotary evaporator to obtain a dry extract; for aqueous extract the water was eliminated with the freeze-drying apparatus. All extracts were stored at 4-8°C until antimicrobial test was assessed.
Estimation of Total Phenolic and Flavonoid Contents
In this work, TPC was estimated using the Folin-Ciocalteu method 28. The Folin-Ciocalteu reagent 1/10 with methanol was prepared which 2.5 mL of this solution was mixed with 0.5 mL of a sample solution. Then 4 mL of sodium carbonate (Na2CO3) (7.5 %) was added. After 30 min of incubation in water bath at 45°C the absorbance was measured at 765nm. TPC was expressed as mg of gallic acid equivalents per g of extract dry weight (mg GAE/g of extract).
The total flavonoid contents (TFC) in the extracts were revealed using the aluminum chloride method. 29 In brief, 0.3 mL of 5% sodium nitrite (NaNO2) was mixed with 5mL containing dissolved sample and distilled water. After 5 min, 0.3 mL of 10% aluminum chloride (AlCl3) was added and the solution was allowed to stand for 6 min. Then, 2 mL of 1 M sodium hydroxide (NaOH) was added and to reach 10 mL the volume was completed with distilled water. The solution was mixed vigorously and allowed to stand for 30 min. The absorbance was measured at 510 nm. The flavonoid content was determined as the rutin equivalent from the calibration curve of rutin standard solutions and expressed as rutin equivalent (mg RE/g of extract).
Antioxidant Activity
Free Radical Scavenging Activity
The radical effect of EOs and extracts was evaluated using the radical 2.2-diphenyl-1-picrylhydrazyl (DPPH) as reported by Huang et al.30, with some modifications. Briefly, the DPPH solution (0.2 mM in methanol) was prepared. Then 2.5 mL of test sample at different concentrations (2.5-100 μg/mL) were added to 0.5 mL of DPPH solution and the absorbance of samples was measured at 517 nm after 30 min. Ascorbic acid and Trolox were used as positive controls.
Ascorbic acid and Trolox were used as positive controls. The calculation of the antioxidant activity was done according to the formula:
DPPH scavenging activity (%) = [(A0 – As) / A0] * 100
Where A0 is the Absorbance of the negative control and As is Absorbance of the test sample at 30 min. The test was carried out in triplicate and the IC50 values were reported as means ± SD.
Reducing power of ferric ions
The reducing activity of EOs and extracts was determined according to the method of Oyaizu.31 The mixture of the 1 ml the phosphate buffer (2.5 mL, 0.2 M, pH 6.6) and the potassium ferricyanide (2.5 mL) was prepared. After incubation for 20 min at 50 °C (water bath), 2.5 mL of trichloroacetic acid (10%) was added to mixture. Then, the solution was centrifuged at 3000Trs/min for 10 min. Finally, 2.5 mL of the supernatant was mixed with 2.5 mL of distilled water and 0.5 mL of 0.1% ferric chloride. Absorbance was measured at 700 nm.
Ascorbic acid (50-450 μg/mL) is used as a standard. The reducing power is expressed in milligram equivalence of ascorbic acid per gram of Essential oil (mg AAE/g of EO).
Antimicrobial Activity
Microorganism Strains and Growth Conditions
To study the antibacterial activity of plant extracts, we have used the following bacteria Listeria innocua CECT 4030, Staphylococcus aureus CECT 976, and Bacillus subtilis DSM 6633, Proteus mirabilis, Escherichia coli K12, and Pseudomonas aeruginosa CECT 118. However, Candida albicans ATCC 10231 was the fungi used to evaluate the antifungal activity.
The pathogen bacterial strains were cultivated in Mueller-Hinton agar (MHA) or Mueller-Hinton Broth (MHB) at 37 °C for 24 h. The fungi were cultured in YPGA medium (5 g yeast extract, 5 g Peptone, 10 g Glucose, 15-18 g Agar, in 1liter) or YPG, and incubated as follows: 48 h at 30°C for fungi;. The inoculum test concentrations are 106 CFU/mL for bacteria and 105 spores/mL for fungi.
Agar Disc Diffusion Method
The antimicrobial activity of C. Ladaniferus and M. Suaveolens extracts and EOs was performed using the agar disc diffusion method.32 Briefly, sterile discs (6 mm diameter) containing 12.5 µL of pure essential oil or 20 µL of ethanolic, methanolic, and aqueous extracts at a concentration of 60 mg/mL dissolved by dimethylsulfoxide (DMSO) 10% were applied onto the surface of the agar-medium which was previously seeded by spreading by the inoculum concentrations test. After incubation, as described above, inhibitory zones were measured. Streptomycin 25 μg and DMSO 10% were used as positive and negative control, respectively.
Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration
The MIC values were determined in sterile 96-well microplate as described by Güllüce et al. 20, with some modifications. First, 100 μL of YPG was distributed in all test wells, except the first well in which a volume of 200 μL containing the essential oil or extracts with a concentration of 25 mg/mL in 10% DMSO. A series of concentrations ranging from 0.097 to 25 mg/mL were prepared by the transfer of 100 μL by scalar dilutions from the first to the ninth well. Then, except the 10th well used as sterility control, 10 μL of the suspension from each well was removed and replaced by the inoculum test concentration as described above. The eleventh well was considered as positive growth control containing only YPG broth. The last well containing 10% DMSO (v/v), without extracts, was used as negative control. Then, the plates were incubated at conditions of growth as described above. After an incubation, a volume of 25 μL of an indicator of microorganism’s growth the was added in each well, the tetrazolium [MTT: 3- (4,5-dimethythiazol) -2-yl-2, 5-diphenyltetrazolium bromide (Sigma)] prepared at a concentration of 0.5 mg/mL in sterile distilled water. The microplate was re-incubated for 30 min at temperature 25°C. Where microbial growth was inhibited, the solution keeps the initial color of MTT. To determine the minimum bactericidal concentration (MBC) value, 10 μL of broth from the uncolored wells was inoculated in LPGA and incubated for 24 h or 72h at 25°C.
Results
Estimation of Phenol and Flavonoid Contents
The estimation of phenol and flavonoid contents are expressed as mg of gallic acid or rutin equivalent per gram, respectively, was presented in Table 1. For C.Ladaniferus, The results showed that the aqueous extract is richer on TPC (76.98 ± 4.6 mg GAE / g of extract) compared to the methanol extract (59.8 ± 4.99 mg GAE/g of extract) and the ethanol extract (53.5 ± 6.28 mg GAE/g extract). However, TFC are only present the aqueous extract (35.51 ± 2.04 mg ER/g of extract). Concerning M. Suaveolens, the methanolic extract showed a high TPC with value of 112.04 ± 2.98 mg GAE/g of extract), followed by the ethanolic extract whose content is in the range of 101.07 ± 4.34 mg GAE/g extract, while 92.70 ± 5.82 mg GAE/g extract are found in the aqueous extract. Furthermore, the content of the methanolic extract in flavonoids and of the order of 99.77 ± 5.4 mg RE/g of extract, followed by a content of 89.02 ± 4.23 mg RE/g of aqueous extract, on the other hand the content found in the ethanolic extract is 68.66 ± 2.94 mg RE/g of extract.
Table 1: Extraction yield, phenolic content of Mentha Suaveolens and Cistus Ladaniferus and antioxidant activity of plant extracts.
| Extracts | Yield | TPC1 | TFC2 | DPPH | FRAP3 | |
| IC50(µg/mL) | ||||||
| Mentha Suaveolens | Ethanol | 1.12±0.02% | 101.07±4.34a | 68.66 ± 2.94 b | 12.57 ± 0.03 ab | 22.45± 1.12 a |
| Methanol | 2.63±0.03% | 112.04±2.98 a | 99.77 ± 5.04 a | 15.83 ± 0.05 bc | 54.44 ± 5.52 a | |
| Aqueous | 3.37±0.12% | 92.70±5.82 a | 89.02 ± 4.23 a | 8.78 ± 0.02 a | 53.03 ± 1,65 b | |
| MSEO | >100 | 5.48±0.6 c | ||||
| Cistus Ladaniferus | Ethanol | 5.67±0.05% | 53.5±6.28b | nd | 26,06 ± 0.05 c | 21.08 ± 0.06 b |
| Methanol | 6.24±0.08 % | 59.8±4.99b | nd | 12.09 ± 0.02 b | 23.69 ± 0.48 b | |
| Aqueous | 6.64±0.06% | 76.98±4.66 a | 35.51±2.04 a | 2.93±0.02 a | 39.53 ± 6.04 a | |
| CLEO | >100 | 0.1 ±0.06 |
1TPC: Total Phenol Content expressed as mg GAE/g of extract.
2TFC: Total flavonoid Content expressed as mg RE/g of extract.
3 Reducing power expressed as mg AAE/g of extract or EO.
MSEO: Mentha Suaveolens essential oil
CLEO: Cistus lafaniferus essential oil
Data are reported to mean (n = 3) ± SD.
Values in the same row of plant column not sharing a common letter (a to c) differ significantly at p<0.05
nd : not detected.
Antioxidant Activity
The results of antiradical activity of C. Ladaniferus and M. Suaveolens were summarized in Table 1. As we can see all extracts exhibit an anti-DPPH in a manner of dose-dependent (Fig. 1 and 2). For C. Ladaniferus, the results showed that the aqueous extract exhibits a significant antiradical effect (IC50 = 2.93 ± 0.02μg/mL), followed by methanolic extract (IC50 = 12.09 ± 0.02μg/mL), and the ethanolic extract reduces the DPPH with IC 50 value of 26.06 ± 0.05μg / mL. However, a low anti-DPPH effect was observed with MSEO with IC50 value superior to 100μg/mL. For M. Suaveolens the results showed that the most important anti-free radical activity is caused by the aqueous extract IC50 = 8.78 ± 0.02μg/mL, followed by ethanol extract IC50 = 12.57 ± 0.03μg/mL, and methanol extract IC50 = 15.83 ± 0.05μg/mL.
The results of FRAP assay, expressed as mg EAA/g, are summarized in Table 1. For C. Ladaniferus the aqueous extract possesses a significant reducing power of 39.53 ± 6.04 mg EAA/g of extract, followed by the methanolic extract with a reducing power of 23.69 ± 0.48 mg EAA/g of extract, and the ethanolic extract with 21.08 ± 0.06 mg EAA / g of extract. One the other hand, among the extracts of M. Suaveolens, the methanolic and aqueous extract have significantly (p <0.05) a very significant reducing power 54.44 ± 5.52 mg EAA / g of extract and 53.03 ± 1.65 mg EAA/g of extract, respectively, compared to the ethanolic extract 22.45 mg EAA/g of extract.
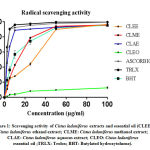 |
Figure 1: Scavenging activity of Cistus Ladaniferus extracts and essential oil |
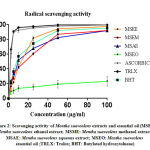 |
Figure 2: Scavenging activity of Mentha Suaveolens extracts and essential oil |
Antimicrobial Activity
The results of antimicrobial effects of extracts and EO of C. Ladaniferus and M. Suaveolens are expressed as diameters of inhibition and the minimal inhibitory concentration and listed in Table 2 and 3. On the hand, CLEO showed a remarkable antibacterial activity against three bacteria tested with variable degree. Indeed, the most sensitive bacteria was P. mirabilis with diameter inhibitory zone value of 25.66±3.05 mm with MIC= 0.19 mg/mL, followed by S. aureus (22±2.64 mm; MIC=6.25 mg/mL), and B. subtilis (15.33±1.52 mm; MIC=0.78 mg/mL). However, a low antifungal was observed against C. albicans (10.66±0.57 mm). For the crude extracts of C. Ladaniferus, the diameters of the zones of inhibition of the antimicrobial effect of the methanolic extract of C. Ladaniferus vary between 10.66 ± 0.57mm and 27 ± 00 mm. Indeed, the methanol extract exhibits an important antibacterial effect against Proteus mirabilis (27 ± 00mm; MIC=3.12 mg/mL), Staphylococcus aureus (20.33 ± 0.57mm; MIC=0.78 mg/mL), with diameters of the zones of inhibition, of, 19.66 ± 0.57mm, respectively. C. albicans was inhibited with a diameter inhibitory zone of 18.33 ± 0.57mm with MIC value of 12.5mg/mL. For the ethanol extract of C. Ladaniferus, the most sensitive bacteria was S. aureus (2.33±0.57mm; MIC=1.56mg/mL), followed by B. subtilis (15.33±0.57 mm; MIC=3.12 mg/mL). While, C. albicans was sensitive to ethanol extract with diameter inhibitory zone value of 16.66±0.57 mm and MIC value of 25 mg/mL. No activity was exhibited by aqueous extract of C. Ladaniferus.
On the other hand, the results indicate the intersting antibacterial activity of M.Suaveolens extracts and EO. In fact, B.subtilis was the most sensitive bacteria to the MSEO with diameter inhibitory zone of 28±2 mm and MIC value of 0.78mg/mL, followed by S.aureus with 19.33±1.52 mm and MIC= 6.25 mg/mL, L. innocua 18.66±1.52 mm and MIC 6.25 mg/mL, and P. mirabilis with 15.33±1.52 mm and MIC=0.78 mg/mL. The MSEO exhibits a good antifungal activity against C. albicans with diameter inhibitory zone value of 49.66±0.57mm and MIC value of 1.56 mg/mL. A low antibacterial activity was observed with ethanol and methanol extracts of M. Suaveolens. no effect of these extracts was observed against fungal tested.
 |
Table 2: Antimicrobial activity of C.Ladaniferus and M.Suaveolens essential oils of by disc diffusion method |
 |
Table 3: Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal (MBC) |
Discussion
The results of our previous ethnopharmacological prospecting of medicinal plants conducted in the Province of Guercif (NE of Morocco) allowed us to appreciate and to know the practices of traditional herbal medicine transmitted by the population of the Province of Guercif, and have constituted a basis of selection of some plants to validate their uses as a remedy.8 The C. Ladaniferus and M. Suaveolens were selected for the estimation of the phenol and flavonoid contents and to evalaute the antioxidant and antimicrobial activities of their extracts and essential oils.
The present study indicates that the extracts of C. Ladaniferus are characterized by a high content of phenol content. These results are similar to previous studies that report a high phenolic content.33 Contrary to our results, Amensour et al.12 reported that the methanolic and ethanolic extracts of C. Ladaniferus have a lower phenol content of 18.43 ± 2.74 mg GAE/g of extract and 11.87 ± 0.53 mg GAE/g of extract. Concerning M. Suaveolens, the high phenol contents of this plant were previously reported.34 Compared with plants of the genus Mentha, the results of other study indicates that the methanolic and aqueous extracts of the species Mentha spicata, Mentha pulegium and Mentha piperita are rich in phenol with contents which vary between 167.2 ± 0.41 and 305.4 ± 1.32μg TAE/mg including aqueous extract of Mentha spicata contains the highest phenol contents.35
The quantitative variation on the phenol contents may be attributed to some parameters such as the chemical structure, the method of extraction, the size of the particles forming the sample, and the solvent.36, 37
There is evidence in the literature that that the antioxidant activity correlates with the phenolic contents. 38, 39 Our results showed a dose-dependent percentage inhibition of DPPH reduction and a high reducing power of extracts of Cistus Ladaniferus in order aqueous>methanol>ethanol extracts; these results may be attributed to the presence of high phenol content in these extracts. Interesting antioxidant results are obtained for M .Suaveolens. Indeed, the aqueous extract showed the most significant anti-free radical activity, followed by the ethanol and methanol extract; knowing that the phenol-rich extracts are in the order methanol> ethanol> aqueous. The missing correlation between the phenol content and antioxidant activity could be explained by numerous factors; (i) the presence of non-phenolic products such as sugars, amino acids which reacts with Folin-Ciocalteu giving a high apparent phenolic level,40,41 (ii) the antagonistic interaction between phenolic and non-phenolic compounds,42 (iii) the antioxidant activity depends on the numbers and position of functional groups.43
For the antimicrobial activity; on the hand, the C. Suaveolens extract and essential oil were active against both gram- positive and gram-negative bacteria. The absence of antibacterial selectivity effect may be attributed to the presence of high content of products known for this propriety in the chemical composition of essential oil, and to the high phenol content in crude extracts. On the other hand, the gram positive strains were most sensitive to the essential oil of M. Suaveolens compared to the gram negative bacteria, these results may be attributed to the presence of phospholipids and lipopolysaccharides in Gram-negative membrane protected them against the external environment.44
Conclusion
This study has displayed that extract of C. Ladaniferus and M. suaveole was possessed antioxidant and antimicrobial activities. Accordingly, we may need more achievement works in future to determine the specific active components which, are responsible for this antioxidant and microbial activity with suggestions for a mechanism of actions.
Acknowledgment
We are very thankful to the Laboratory of Biology and Health, Sciences Faculty of Tetouan and the Laboratory of Agri-Food and Health of FST-Settat, for providing us with the microorganisms used in this study.
Conflict of Interest
There is no conflict of interest.
Funding
There is not funding source
References
- Bouyahya A, Abrini J, Et-Touys A, Bakri Y, Dakka N. Indigenous knowledge of the use of medicinal plants in the North-West of Morocco and their biological activities. European Journal of Integrative Medicine.; 13: 9-25 (2017).
- Wang Y-Z, Fu S-G, Wang S-Y, Yang D-J, Wu Y, Chen Y-C. Effects of a natural antioxidant, polyphenol-rich rosemary (Rosmarinus officinalis L.) extract, on lipid stability of plant-derived omega-3 fatty-acid rich oil. LWT – Food Science and Technology.; 89:210-216 (2018).
- Ahmed AF, Attia FAK, Liu Z, Li C, Wei J, Kang W. Antioxidant activity and total phenolic content of essential oils and extracts of sweet basil (Ocimum basilicum) plants. Food Science and Human Wellness.; 8(3): 299-305 (2019).
- Cai M, Lv H, Cao C, Zhang L, Cao R, Xu B. Evaluation of antimicrobial activity of Pterocarpus Industrial Crops and Products.; 140: 111668 (2019).
- Amrani A, Mecheri A, Bensouici C, Boubekri N, Benaissa O, Zama D, Benayache F, Benayache S. Evaluation of antidiabetic, dermatoprotective, neuroprotective and antioxidant activities of Chrysanthemum fontanesii flowers and leaves extracts. Biocatalysis and agricultural biotechnology.; 20: 101-209 (2019).
- Tuama AA, Mohammed AA. Phytochemical screening and in vitro antibacterial and anticancer activities of the aqueous extract of Cucumis sativus. Saudi Journal of Biological Sciences.; 26(3): 600-604 (2019).
- Olaokun OO, Alaba AE, Ligege K, Mkolo NM. Phytochemical content, antidiabetes, anti-inflammatory antioxidant and cytotoxic activity of leaf extracts of Elephantorrhiza elephantina (Burch.) Skeels. South African Journal of Botany.; 128: 319-325 (2020).
- Khabbach A, Libiad, M, Ennabili, A, Bousta, D. Medicinal and cosmetic use of plants from the province of Taza, Northern Morocco. Boletin Latinoamericano y del Caribe de Plantas Medicinales y Aromaticas.; 11: 46-60 (2012).
- Benali T, Khabbach A, Ennabili A, Hammani K. Ethnopharmacological prospecting of medicinal plants from the Province of Guercif (NE of Morocco). Moroccan Journal of Biology.; 14: 1-14 (2017).
- Mariotti JP, Tomi F, Casanova J, Costa J, Bernardini AF. 1997. Composition of the Essential Oil of Cistus Ladaniferus Cultivated in Corsica (France). Flavour and Fragrance Journal. ; 12: 147-151 (1997).
- Chaves N, Rı́os JJ, Gutierrez C, Escudero JC, MOlı́as J. Analysis of secreted flavonoids of Cistus ladanifer by high performance liquid chromatography–particle beam mass spectrometry. Journal of Chromatography A.; 799: 111–115 (1998).
- Amensour M, Sendra E, Pérez-Alvarez JA, Skali-Senhaji N, Abrini J, Fernández-López J. Antioxidant Activity and Chemical Content of Methanol and Ethanol Extracts from Leaves of Rockrose (Cistus Ladaniferus). Plant Foods Hum Nutr.; 65:170–178 (2010).
- Rossi P-G, Berti L, Panighi J, Luciani A, Maury J, Muselli A, Serra DR, Gonny M, Bolla J-M. Antibacterial Action of Essential Oils from Corsica. Journal of Essential Oil Research.; 19 (2): 176-182 (2007).
- Andrade D, Gil C, Breitenfeld L, Domingues F, Duarte AP. Bioactive extracts from Cistus ladanifer and Arbutus unedo Industrial Crops and Products.; 30: 165–167 (2009).
- Bouamama H, Noel T, Villard J, Benharref A, Jana M, 2006. Antimicrobial activities of the leaf extracts of two Moroccan Cistus species. Journal of Ethnopharmacology.; 104: 104–107 (2006).
- Barros L, Dueñas M, Tiago-Alves C, Silva S, Henriques M, Santos-Buelga C, Ferreira I. Antifungal activity and detailed chemical characterization of Cistus ladanifer phenolic extracts. Industrial Crops and Products.; 41: 41–45 (2013).
- Barrajón-Catalán E, Fernández-Arroyo S, Saura D, Guillén E, Fernández-Gutiérrez A, Segura-Carretero A, Micol V. Cistaceae aqueous extracts containing ellagitannins show antioxidant and antimicrobial capacity, and cytotoxic activity against human cancer cells. Food Chemical. Toxicology.; 48: 2273–2282 (2010).
- Bellakhdar J, Claisse R, Fleurentin J, Younos C. Repertory of standard herbal drugs in the Moroccan pharmacopoeia. Journal of Ethnopharmacology.; 35: 123-143 (1991).
- El-Hilaly J, Hmammouchi M, Lyoussi B. Ethnobotanical studies and economic evaluation of medicinal plants in Taounate province (Northern Morocco). Journal of Ethnopharmacology. 86: 149–158 (2003).
- Güllüce M, Sahin F, Sokmen M, Ozer H, Daferera D, Sokmen A, Polissiou M, Adiguzel A, Ozkan H. Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia L. ssp. longifolia. Food Chemistry. 103: 1449–1456 (2007).
- Zaidi F, Voirin B, Jay M, Viricel MR. Free flavonoid aglycones from leaves of Mentha pulegium and Mentha Suaveolens (labiatae). Phytochemistry.; 48:991-994 (1998).
- Božović M, Pirolli A, Ragno R. Mentha Suaveolens Ehrh. (Lamiaceae) Essential Oil and Its Main Constituent Piperitenone Oxide: Biological Activities and Chemistry. Molecules.; 20: 8605-8633 (2015).
- Rasooli I, Rezaei MB. Bioactivity and Chemical Properties of Essential Oils from Zataria multiflora Boiss and Mentha longifolia (L.) Huds. Journal of Essential Oil Research.; 14 (2): 141-146 (2002).
- Mahboubi M, Haghi G. Antimicrobial activity and chemical composition of Mentha pulegium essential oil. Journal of Ethnopharmacology.; 119: 325–327(2008).
- Hussain AI, Anwar F, Nigam PS, Ashraf M, Gilani AH. Seasonal variation in content, chemical composition and antimicrobial and cytotoxic activities of essential oils from four Mentha Journal of the Science of Food and Agriculture.; 90: 1827–1836 (2010).
- Derwich E, Benziane Z, Boukir A. 2010. GC/MS analysis and antibacterial activity of the essential oil of Mentha pulegium grown in Morocco. Research Journal of Agriculture and Biological Sciences.; 6 (3):191-198 (2010).
- de Sousa Barros A, de Morais SM, Ferreira PAT, Vieira ÍGP, Craveiro AA, Fontenelle ROS, de Menezes IESA, da Silva FWF, de Sousa HA. Chemical composition and functional properties of essential oils from Mentha Industrial Crops and Products.; 76 : 557–564 (2015).
- Lister E, Wilson P. Measurement of Total Phenolics and ABTS Assay for Antioxidant Activity. Crop Research Institute, Lincoln, New Zealand, (2001).
- Dewanto V, Wu XZ, Liu RH. Processed sweet corn has higher antioxidant activity. Dewanto, V., Wu, X., & Liu, R. H. (2002). Processed Sweet Corn Has Higher Antioxidant Activity. Journal of Agricultural and Food Chemistry.; 50(17): 4959–4964 (2002).
- Huang B, Ke H, He J, Ban, Zeng H, Wang Y. Extracts of Halenia elliptica exhibit antioxidant properties in vitro and in vivo. Food and Chemical Toxicology.; 49(1): 185–190 (2011).
- Oyaizu M. Studies on product of browning reaction prepared from glucose amine. The Japanese Journal of Nutrition and Dietetics.; 44: 307–15 (1986).
- Rota C, Carramiñana JJ, Burillo J, Herrera A. In vitro antimicrobial activity of essential oils from aromatic plants against selected foodborne pathogens. Journal of Food Protection.; 67: 1252–1256 (2004).
- Zidane H, Elmiz M, Aouinti F, Tahani A, Wathelet J, Sindic M, Elbachiri A. Chemical composition and antioxidant activity of essential oil, various organic extracts of Cistus ladanifer and Cistus libanotis growing in Eastern Morocco. African Journal of Biotechnology.; 12 (34): 5314-5320 (2013).
- Moldovan RI, Oprean R, Benedec D, Hanganu D, Duma M, Oniga I, Vlase L. LC-MS analysis, antioxidant and antimicrobial activities for five species of mentha cultivated in Romania. Digest Journal of Nanomaterials and Biostructures.; 9(2): 559–566 (2014).
- Barchan A, Bakkali M, Arakrak A, Pagán R, Laglaoui A. The effects of solvents polarity on the phenolic contents and antioxidant activity of three Mentha species extracts. International Journal of Current Microbiology and Applied Sciences.; 3(11): 399-412 (2014).
- Trosznska A, Esterella I, Amores MLL, Hernandez T. Antioxidant activity of pea (Pisum sativum) seed coat acetone extract. LWT – Food Science and Technology.; 35(2): 158-164 (2002).
- Naczk M, Shahidi F. Extraction and analysis of phenolics in food. Journal of Chromatography A.; 1054(1-2): 95-111 (2004).
- Kim DO, Chun OK, Kim YJ, Moon HY, Lee CY. Quantification of polyphenolics and their antioxidant capacity in fresh plums. Journal of Agricultural and Food Chemistry.; 51(22): 6509–6515 (2003).
- Turkmen N, Sari F, Velioglu YS. Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin–Ciocalteu methods. Food Chemistry.; 99(4); 835–841 (2006).
- Prior RL, Wu X, Schaich K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chemistry.; 53(10) :4290-4302 (2005).
- Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P, Vidal N. Antioxidant activity of some algerian medicinal plants extracts containing phenolic compounds. Food Chemistry.; 97: 654–660 (2006).
- Babbar N, Oberoi HS, Uppal DS, Patil RT. Total phenolic content and antioxidant capacity of extracts obtained from six important fruit residues. Food Research International.; 44: 391–396 (2011).
- Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chemistry.; 99:191–203 (2006).
- Saunders NA, Lee MA. Real-time PCR: Advanced Technologies and Applications. Horizon Scientific Press, Salisbury.; (2013).








