Purnamanita1 , Budu2
, Budu2 , Mochammad Hatta3, Muhammad Nasrum Massi3
, Mochammad Hatta3, Muhammad Nasrum Massi3 , Rosdiana Natzir4
, Rosdiana Natzir4 , A. M. Ichsan2
, A. M. Ichsan2 and Hidayat Sujuti5
and Hidayat Sujuti5
1Public Eye Health Centre Makassar, Indonesia
2Department of Ophthalmology Faculty of Medicine Hasanuddin University Makassar, Indonesia
3Molecular Biology and Immunology Laboratory, Faculty of Medicine, Hasanuddin University Makassar, Indonesia
4Department of Biochemistry, Faculty of Medicine Hasanuddin University Makassar, Indonesia
5Department of Ophthalmology, Faculty of Medicine Brawijaya University Malang, Indonesia
Corresponding Author E-mail : hattaram@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1916
Abstract
Objective: To evaluate the effectiveness of 20 mg subconjunctival injections of Triamcinolone and their relationship to changes in VEGF mRNA expression on the risk of postoperative recurrence Methods: Ten patients with stage II pterygium were divided into two groups, each group consisted of 5 samples given a subconjunctival injection of 20 mg Triamcinolone, and 5 samples given a placebo injection containing Balance Salt Solution (BSS), and venous blood was taken one week before excision. One week after injection, pterygium excision was performed using conjunctival autograft technique. One month postoperatively, VEGF mRNA expression was examined by taking venous blood and clinical changes after the injection was observed.Results: From 10 samples of pterygium patients, changes of expression in VEGF mRNA in the Triamcinolone group were 3.94 ± 0.96 and in the placebo group, the expression in VEGF mRNA was 1.27 ± 0.31, p = 0.006. The expression of mRNA in pterygium tissue in the Triamcinolone group was 8.98 ± 1.04 and in the placebo group it was 7.75 ± 0.62, p = 0.07. Clinical features appeared to be more minimal in the triamcinolone group than in the placebo group.Conclusion: The administration of subconjunctival injection of 20 mg Triamcinolone and the excision with conjunctival autograft is more effective in suppressing postoperative inflammation. It means that the likelihood of post-excision recurrence risk is smaller in this group compared to placebo
Keywords
mRNA VEGF; Pterygium; Recurrence; Triamcinolone
Download this article as:| Copy the following to cite this article: Purnamanita P, Budu B, Hatta M, Massi M N, Natzir R,Ichsan A. M, Sujuti H. The Effectiveness of Triamcinolone Injection on Risk of Postoperative Operations with the Conjunctiva Autograft Technique and Its Association with Change of VEGF mRNA Expression. Biomed Pharmacol J 2020;13(2). |
| Copy the following to cite this URL: Purnamanita P, Budu B, Hatta M, Massi M N, Natzir R,Ichsan A. M, Sujuti H. The Effectiveness of Triamcinolone Injection on Risk of Postoperative Operations with the Conjunctiva Autograft Technique and Its Association with Change of VEGF mRNA Expression. Biomed Pharmacol J 2020;13(2). Available from: https://bit.ly/2LZKpiq |
Introduction
Pterygium is fibrovascular dysplasia in the triangular bulb conjunctiva, usually located in the nasal part of the conjunctiva1. Growth of conjunctival tissue is triggered by inflammation due to chronic eye irritation. Generally, pterygium is asymptomatic, but this can result in inflammation, invasive and tissue proliferation and postoperative recurrence2. Pterygium can interfere the vision when it covers the pupil, but patients are usually operated on for cosmetic reasons3. Handling pterygium in the form of surgery, several techniques are used such as bare sclera, autologous conjunctival graft or with amniotic membrane4.
Corticosteroids have long been used in ocular inflammation, which can be given in topical, subconjunctival, intraocular and systemic forms. Triamcinolone is one of the most commonly used corticosteroids, available in two forms, acetonide and diacetate, which is an intermediate-acting corticosteroid that has a working period of up to 6 weeks when injected5. Triamcinolone acetone is the most widely used drug worldwide. Triamcinolone injection in the eye is available in suspension form, can be given periocularly or intraocularly. The administration of triamcinolone injection has been reported in cases of Anterior scleritis, Corneal graft rejection, Vernal keratoconjunctivitis, Uveitis, Episcleritis, Pterygium surgery, Superior limbic keratoconjunctivitis, Corneal graft , Herpetic keratitis, Intraocular surgery, Thyroid eye disease, and Neovascularization5.
The main therapy in pterygium is excision surgery, can be in the form of a simple excision (bare-sclera technique) or excision of the graft (conjunctival graft or amnion graft). Postoperative recurrence can occur. Recurrence of pterygium is characterized by proliferation and migration of fibroblasts. To prevent recurrence, fibrovascular activity as a sign of recurrence impending must be stopped immediately, one of which is by giving intralesional injection6. Triamcinolone is an intermediate-acting steroid that plays a role in decreasing fibroblast activity, formation of new blood vessels and inflammation. The technique of intralesional administration as an adjuvant therapy gives higher concentrations directly to the fibrovascular area, thereby providing a better inhibitory effect and lower recurrence compared to topical steroids7. Intralesional therapy does not only inhibit recurrence but also improves the appearance of areas that are exposed to. Conjunctival injection, thickening and size of fibrovascular tissue are generally smaller in the triamcinolone group.
Triamcinolone acetonide inhibits angiogenesis directly through the influence of vascular endothelial cells and indirectly through other cells released by various cytokines that cause angiogenesis8. There are 2 types of corticosteroid injections; the short-acting type such as dexamethasone and long-acting type such as triamcinolone and hydrocortisone. Triamcinolone is soluble in water and has an effect of 15-21 days. The use of subconjunctival corticosteroids in preventing recurrence has not been explored either9. VEGF is a key in the process of physiological angiogenesis during embryogenesis, bone growth and reproductive function, but also plays a role in the pathological angiogenesis process such as tumor growth, intraocular neovascular syndrome10. VEGF plays a role in diseases in the anterior segment, as in pterygium. Corneal neovascularization can lead to impaired visual acuity and determine caused by inflammation and corneal scaring11. VEGF affects the increase in vascular permeability, angiogenesis and lymphangiogenesis, which causes an increase in VEGF expression, which causes neovascularization and inflammation in the pterygium12. The purpose of this study was to assess how effective the administration of subconjunctival injection of 20 mg Triamcinolone to Vascular Endothelial Growth Factor (VEGF) mRNA expression, in blood and pterygium tissue in pterygium patients operated on by conjunctival autograft techniques.
Material and Methods
This research was conducted after obtaining approval from the ethics committee. Every subject included in the study was recorded for the identity and made no objections made to be included in this study (informed consent). In this study, all actions were taken with the consent of the subjects through an informed consent sheet, after the subjects received an explanation of the actions to be taken, and after the study was declared to meet ethical requirements to be carried out from the Ethics Commission on Human Health Research at the Faculty of Medicine, Hasanuddin University, RSUH and RSWS Makassar . Ten eyes out of ten people suffering from pterygium who met the inclusion criteria included in this study. The inclusion criteria included patients aged 30-45 years who were diagnosed with stage II primary pterygium according to Bharvaga criteria and did not suffer from systemic diseases which were contraindicated for excision such as not suffering from diabetes mellitus or hypertension. As for exclusionary criteria, ie suffering from pseudopterygium, suffering from an infectious disease of the eye, pterygium excision had been performed before, had only one eye to see and which was not cooperative during sampling. Age and sex of each study subject were recorded, visual acuity in both eyes was examined, slit lamp examination was performed to determine the type of pterygium and its degree / stage, as well as examination of eyeball pressure, blood pressure examination and random blood sugar (RBS) examination was performed and all examination results above were recorded.
In this study, venous blood of all patients was taken for examination of Vascular Endothelial Growth Factor (VEGF) mRNA expression before injection. Then, the patients were divided into 2 groups, 5 people received subconjunctival injection of 20 mg Triamcinolone acetate (Flamicort 40 mg, vial @1 ml), and 5 people received subconjunctival injection of Balanced Salt Solution. Everything that happened as a result of the injection was recorded. All actions were carried out under an operating microscope. One week after injection, pterygium excision surgery was performed. The surgical procedure was carried out under an operating microscope. After speculum placement, anesthesia was performed by subconjunctival injection of 2% Lidocain. The pterygium head was released from the cornea by using a knife. The fibrovascular tissue that lies beneath the conjunctiva was carefully removed and cut with tenotomy scissors. The sclera and cornea were cleaned from the remaining pterygium using a curette. Autolimbal conjunctival graft was performed with a size corresponding to the excised tissue. At the end of the surgery, antibiotic and dexamethasone eye ointment (Cendo Xitrol) were given. Post surgery, antibiotic and steroid eye drops (Cendo Xitrol) and lubricant eye drops (Cendo Lyteers) were given. Samples of the pterygium that had been taken were then placed in transport media and stored in an ice box to be taken to the laboratory and stored in the -20°C for real-time PCR examination. The excised tissue was checked for Vascular Endothelial Growth Factor (VEGF) mRNA expression levels.
Postoperative evaluation on day 1, week 1, and one month after surgery is done while assessing signs of postoperative recurrence. One month after excision another venous blood was taken to assess the Vascular Endothelial Growth Factor (VEGF) levels. Examination of mRNA of VEGF gene in blood and pterygium tissue by real-time PCR according to previous study done by first extracting nucleic acid, then RNA amplification is done using the primers VEGF for: 5′-GCA CCC ATG GCA GAA GG-3′and VEGF Rev: 5′-CTC GAT TGG ATG GCA GTA GCT-3′, while Housekeeping gene are β-actin for: 5′-CGC CCA GCA CGA TGA AA-3′β-actin rev: 5′-CCG CCG ATC CAC ACA GA-3′. The target gene’s mRNA expression profile was determined and after that the calibration curve with cycle threshold (Ct) was calculated and the relative quantization of target gene expression was evaluated using the Ct comparison method12,13. All data obtained were grouped according to the purpose and type of data. Univariate analysis was carried out on each of the variables studied to determine the description of the frequency distribution and data normality of all research variables. Bivariate analysis was performed to determine the relationship between variables, if the normal data distribution used the Independent Sample T Test and Paired T test, whereas if the data distribution was not normal using the Wilcoxon test and multivariate analysis, using the Anova test. Statistical analysis using the IBM SPSS program version 23.0 with a degree of confidence of 95% and a value of α ≤ 0.05,
Results and Discussions
From the 10 samples studied, it was found that the age of the sample was 31-45 years, there were 7 women and 3 men, had lower secondary education, as many as those who worked and those who did not work. The change in VEGF mRNA expression between the Triamcinolone and placebo groups can be seen in Table 1.
Table 1: Comparison of changes in VEGF mRNA expression between the Triamcinolone and placebo groups
| VEGF mRNA expression | N | Mean | Std. Deviation | Std. Error | 95% Confidence Interval for Mean | Minimum | Maximum | |
| Lower Bound | Upper Bound | |||||||
| Triamcinolone (blood) | 5 | 3.9444 | 0.96316 | 0.43074 | 2.7484 | 5.1403 | 2.87 | 4.81 |
| Placebo (blood) | 5 | 1.27 | 0.31061 | 0.13891 | 0.8843 | 1.6557 | 0.96 | 1.76 |
| Triamcinolone (tissue) | 5 | 8.9874 | 1.04367 | 0.46674 | 7.6915 | 10.2833 | 7.3 | 9.83 |
| Placebo (tissue) | 5 | 7.7555 | 0.6259 | 0.27991 | 6.9783 | 8.5326 | 6.91 | 8.56 |
In Table 1, changes in VEGF mRNA expression in blood samples showed significant results in the Triamcinolone group, where VEGF mRNA expression was higher than in the placebo group, which was 3.94 ± 0.96 vs. 1.27 ± 0.31, p = 0.006. As for tissue samples, VEGF mRNA expression in the Triamcinolone group was also higher than in the placebo group, which was 8.98 ± 1.04 in the Triamcinolone group, and in the placebo group 7.75 ± 0.62, p = 0.07 (Figure 1)
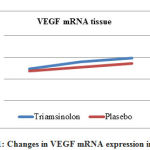 |
Figure 1: Changes in VEGF mRNA expression in tissues |
The clinical picture after injection in the Triamcinolone and placebo groups can be seen in the following figure (Figures 2 and 3).
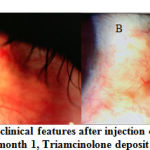 |
Figure 2: Changes in clinical features after injection of Triamcinolone |
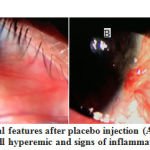 |
Figure 3: Changes in clinical features after placebo injection |
Discussion
From the research that had been done, the result was higher in expression in the Triamcinolone group compared to the placebo group and was statistically significant in blood samples, whereas in tissue samples the results obtained were not significant. The placebo group seems higher than in Triamcinolone group due to baseline sample, but mRNA VEGF expression decrease more higher than in placebo group one month after injection.In this study, significant results were obtained in the group given the injection of Triamcinolone, 3.94 ± 0.96 vs 1.27 ± 0.31, p = 0.006. Triamcinolone reduced inflammation and therefore angiogenesis and neovascularization due inhibit to inflammation and VEGF not release from their receptor. This result was accordance with that obtained by Tong, 2006, who found that Triamcinolone inhibits the effect of angiogenesis by reducing VEGF from VEGF receptors. VEGF plays a role in the pathological angiogenesis process such as tumor growth, intraocular neovascular syndrome such as in pterygium10. VEGF affects the increase in vascular permeability, angiogenesis and lymphangiogenesis, which causes an increase in VEGF expression, which causes neovascularization and inflammation in the pterygium14,15. VEGFR expression was significantly higher in pterygium than in the conjunctiva, high expression in pterygium compared to normal control and was associated with cell proliferation and angiogenesis in pterygium. VEGF plays a role in the formation of pterygium and contributes to the ability of pterygium angiogenesis. In addition, it was also found that the therapeutic response to antiangiogenesis drugs might depend on the variant type of VEGF allele16. Tong et al. found that triamcinolone reduced VEGF mRNA expression in RPE cells in choroidal neovascularization and macular edema cases. It is suspected that triamcinolone inhibits the effects of angiogenesis from VEGF receptors by suppressing the production of VEGF or triamcinolone strengthens the expression of PEDF which is a potent inhibitor of angiogenesis17.
In this study, triamcinolone injection was only given once, one week before excision, and the expression of VEGF mRNA in the blood was lower than expression in pterygium tissue. Inflammatory process occurs in pterygium tissue and the injection will increase the inflammatory process that already exist, so, mRNA VEGF expression in tissue sample was higher than in the blood. A study by Kang et al. found that triamcinolone was not potent as an anti-VEGF therapy in preventing neovascularization of the cornea. The inhibitory effect was obtained if used together with Bevacizumab. The efficacy of steroids in suppressing VEGF expression was lower than the direct mechanism of anti-VEGF, hence the combination of triamcinolone and bevacizumab makes a greater inhibitory effect than single anti VEGF. VEGF itself plays a major role in angiogenesis while steroids have a smaller role. However, the combination of triamcinolone and bevacizumab did not provide significant results compared to bevacizumab therapy alone in the management of corneal neovascularization18. VEGF mRNA can be analyzed by RT-PCR, realtime PCR, Elisa and Western blots. Sava et al. obtained 40% of pterygium samples having high mRNA amplification19. High levels of VEGF mRNA detected in basal cells could be explained by phenotypes and specific behaviors as explained by Chui et al. which says that basal cells have premalignant features20. In the clinical picture 1 month after injection as shown in Tables 2 and 3, it appeared that the conjunctiva was still hyperemic in the placebo group, whereas in the Triamcinolone group there was a picture of hyperemia limited by triamcinolone deposits. Athanasiadis states that the injection of local Triamcinolone suppressed the immune response for some time, due to the natural crystalline nature of this drug5. The presence of this substance for several months in the body locally makes this drug classified as intermediate glucocorticoids, its strength is 4x stronger than hydrocortisone and 5x stronger than betamethasone21. Parenterally, the biological half-life of triamcinolone is 18-36 hours22. Kalina, 1995 reported that triamcinolone deposits can last up to 13 months after injection23.
Also, Prabhasawat (2006) reported that pterygium fibrovascular tissue had not developed optimally until 6 months postoperatively, so that if given before perfect proliferation occurs, it would give better results, reducing the use of excessive drugs that risked increased complications. Intralesional injection of 5 FU with triamcinolone was more effective in preventing impending recurrence compared with only topical steroids6. The most efficient subconjunctival injection of Triamcinolone was given by the technique of making white crystal precipitation and removing the supernatant before injection, this could be done if placing the insulin with upright position and taking into account the crystal separation from the solvent. If 1 ml of triamcinolone is given, a precipitate of 0.1 – 0.15 ml will be obtained. The presence of white areas at the time of injection in the subconjunctiva showed the formation of the drug deposits, so that absorption through the cornea can be longer and the risk of side effects from preservatives can be minimized5,24. The weakness of this study is that the subjects studied were small in number, that was, only 10 eyes, no data on pterygium tissue before surgery due to ethical factors, and only postoperative tissue was analyzed, so there was no comparison between expression before and after tissue injection. In addition, follow-up and examination of VEGF mRNA expression was not long enough; only take up to 1 month after injection, so that the risk of recurrence cannot be further analyzed, although it can be expected that the greatest risk of recurrence is in the placebo group.
Conclusion
Expression of mRNA VEGF decreased after the administration of subconjunctival injection of 20mg Triamcinolone with conjunctival autograft effective in suppressing postoperative inflammation.
Acknowledgement
Thanks to all registered patients at Public Eye Health Centre Makassar, Indonesia who participated in the study.
Conflict of interest
The authors of this paper declare that there are no conflicts of interest.
Funding Source
This paper received no grants from any funding agency or sectors.
Reference
- Nangia V, Jonas JB, Nair D, Saini N, Nangia P, Panda-Jonas S. Prevalence and associated factors for pterygium in rural agrarian Central India. The Central India Eye and Medical Study. PLoS One. 2013;8(12):1–6.
- Themen HCI, Mans DRA, Bipat R, Doelwijt DJ, Jiawan D, de Mesquita-Voigt ATB. Possible correlation of plica-limbal distance with the presence of primary medial pterygium. Transl Biomed. 2010;1(2).
- Fotouhi A, Hashemi H, Khabazkhoob M, Mohammad K. Prevalence and risk factors of pterygium and pinguecula: The Tehran Eye Study. Eye. 2009;23(5):1125–9.
- Alpay A, Uǧurbaş SH, Erdoǧan B. Comparing techniques for pterygium surgery. Clin Ophthalmol. 2009;3(1):69–74.
- Athanasiadis Y, Tsatsos M, Sharma A, Hossain P. Subconjunctival triamcinolone acetonide in the management of ocular inflammatory disease. J Ocul Pharmacol Ther. 2013;29(6):516–22.
- Prabhasawat P, Tesavibul N, Leelapatranura K, Phonjan T. Efficacy of Subconjunctival 5-Fluorouracil and Triamcinolone Injection in Impending Recurrent Pterygium. Ophthalmology. 2006;113(7):1102–9.
- Hill JC. Pathogenesis of pterygium. Eye. 1989;3(2):218–26.
- Murata M, Shimizu S, Horiuchi S, Taira M. Inhibitory effect of triamcinolone acetonide on corneal neovascularization. Graefe’s Arch Clin Exp Ophthalmol. 2006;244(2):205–9.
- Singh S, Pal V, Dhull C. Supratarsal injection of corticosteroids in the treatment of refractory vernal keratoconjunctivitis. Indian J Ophthalmol. 2001 Oct 1;49(4):241–5.
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–76.
- Cornel S, Adriana ID, Mihaela TC, Speranta S, Algerino DS, Mehdi B, et al. Anti-vascular endothelial growth factor indications in ocular disease. Rom J Ophthalmol. 2015;59(4):235–42.
- Ang Yuan, Chong-Jen Yu, Kwen-Tay Luh, Wen-Jone Chen, Fang-Yue Lin, Sow-Hsong Kuo, and Pan-Chyr Yang. 2000. Quantification of VEGF mRNA Expression in Non-Small Cell Lung Cancer Using a Real-Time Quantitative Reverse Transcription-PCR Assay and a Comparison with Quantitative Competitive Reverse Transcription-PCR. Laboratory Investigation. 80(11):1671-1680.
- Hatta M, Surachmanto EE, Islam AA, Wahid S. 2017. Expression of mRNA IL-17F and sIL-17F in atopic asthma patients. BMC Research Notes.10:202. DOI: 10.1186/s13104-017-2517-9
- Zhou WP, Zhu YF, Zhang B, Qiu WY, Yao YF. The role of ultraviolet radiation in the pathogenesis of pterygia (Review). Mol Med Rep. 2016;14(1):3–15.
- Witmer AN, Vrensen GFJM, Van Noorden CJF, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Progress in Retinal and Eye Research. 2003.
- Peng ML, Tsai YY, Tung JN, Chiang CC, Huang YC, Lee H, et al. Vascular endothelial growth factor gene polymorphism and protein expression in the pathogenesis of pterygium. Br J Ophthalmol. 2014;98(4):556–61.
- Tong JP, Lam DSC, Chan WM, Choy KW, Chan KP, Pang CP. Effects of triamcinolone on the expression of VEGF and PEDF in human retinal pigment epithelial and human umbilical vein endothelial cells. Mol Vis. 2006;12(September 2005):1490–5.
- Kang S, Chung SK. The effect of subconjuctival combined treatment of bevacizumab and triamcinolone acetonide on corneal neovascularization in rabbits. Cornea. 2010;29(2):192–6.
- Sava MGP, Raica ML, Cimpean AMG. VEGF mRNA assessment in human pterygium: A new “scope” for a future hope. Ophthalmic Res. 2014;52(3):130–5.
- Chui J, Coroneo MT, Tat LT, Crouch R, Wakefield D, Di Girolamo N. Ophthalmic pterygium: A stem cell disorder with premalignant features. Am J Pathol. 2011;178(2):817–27.
- Gaudio PA. A review of evidence guiding the use of corticosteroids in the treatment of intraocular inflammation. Ocul Immunol Inflamm. 2004;12(3):169–92.
- Cárdenas-Cantú E, Zavala J, Valenzuela J, Valdez-García JE. Molecular Basis of Pterygium Development. Semin Ophthalmol. 2016;31(6):567–83.
- Kalina PH, Erie JC, Rosenbaum L. Biochemical Quantification of Triamcinolone in Subconjunctival Depots. J Chem Inf Model. 2013;53(9):1689–99.
- Ober MD, Barile GR, Tari SR, Tossi GM, Schiff WM, Chang S. Measurement of the Actual Dose of Triamcinolone Acetonide Delivered by Common Techniques of Intravitreal Injection. Am J Ophthalmol. 2006;142(4):0–4.








