Shanmugapriya S1*, Saravanan T2, Saravanan A3, Yamuna Devi MS1 and Kishore V3
1Department of Pharmacology, PSG Institute of Medical Sciences and Research, Peelamedu, Coimbatore, Tamilnadu, India
2Department of Medicine, PSG Institute of Medical Sciences and Research, Peelamedu, Coimbatore, Tamilnadu, India
3Resident, PSG Institute of Medical Sciences and Research, Peelamedu, Coimbatore, Tamilnadu, India
Corresponding Author E-mail : somasundaram999@rediffmail.com
DOI : https://dx.doi.org/10.13005/bpj/1857
Abstract
Gliptins are drugs that inhibit the enzyme dipeptidyl peptidase-4 and are one of the current treatment options for type 2 diabetes mellitus. The study aimed to compare the lipid lowering effects of teneligliptin with simvastatin in diet induced hyperlipidemic rats.22 Sprague Dawley male rats were administered high fat diet over a period of 4 weeks to induce hyperlipidemia, after which, 10 rats each in groups 1 and 2wereorally gavaged with simvastatin (standard) and teneligliptin (treatment) respectively for 2 weeks. 2 rats in group 3 were vehicle controls. Blood samples were collected at baseline, at the end of 4 weeks and 6 weeks to determine the levels of total cholesterol, triglycerides, low density lipoprotein cholesterol and high density lipoprotein cholesterol. Histopathology of liver and aorta of 2 control animals and 3 animals each from the groups 1 and 2 was done at the end of the study.A paired t test showed a statistically significant reduction in mean total cholesterol (p<0.001), triglycerides (p<0.001) and low density lipoprotein cholesterol (p=0.003), but no significant change in mean high density lipoprotein cholesterol (p=0.796) in teneligliptin group at the end of 6 weekscompared to the pre-treatment values. There was no statistically significant difference between the simvastatin and teneligliptin groups in all the four lipid parameters studied. Compared to baseline, there was a rise in body weight after induction and a reduction post-treatmentin both groups which did not achieve statistical significance. Histopathology of liver in both groups 2 and 3 demonstrated reversal of the congestion and mild fatty changes which were apparent in the control group while sections of aorta failed to reveal any significant changes even in the control group.The lipid lowering property of teneligliptin demonstrated in this study will be therapeutically beneficial in type 2 diabetic patients with dyslipidemia.
Keywords
Dipeptidyl Peptidase4 Inhibitors; Dyslipidemia; Hypolipidemic Activity; Teneligliptin
Download this article as:| Copy the following to cite this article: Shanmugapriya S, Saravanan T, Saravanan A, Devi MS. Y, Kishore V. Lipid Lowering Effect of Teneligliptin in Comparison to Simvastatin in Diet Induced Hyperlipidemic Rats. Biomed Pharmacol J 2020;13(1). |
| Copy the following to cite this URL: Shanmugapriya S, Saravanan T, Saravanan A, Devi MS. Y, Kishore V. Lipid Lowering Effect of Teneligliptin in Comparison to Simvastatin in Diet Induced Hyperlipidemic Rats. Biomed Pharmacol J 2020;13(1). Available from: https://bit.ly/2vwabpE |
Introduction
Hyperlipidemia is a metabolic disorder that involves abnormally increased levels of lipids and lipoproteins in the blood. Most dyslipidemias (80%) are related to diet and lifestyle, although familial disorders (20%) are important as well. The American Heart Association estimates a prevalence of 11.9% which is around28.5 million adults ≥20 years of age having serum total cholesterol levels ≥240 mg/dl, which is considered a high level necessitating treatment.1 A study conducted by Indian Council of Medical Researchshows a high prevalence in India with approximately 79% of the 16607 study subjects manifesting abnormalities inat least one of the lipid parameters.2
Hyperlipidemia is one of the major risk factors that causearteriosclerosis, cerebral stroke, coronary heart disease, myocardial infarction and renal failure. Statins are widely used to lower cholesterol levels because of their inhibitory effects on the 3-hydroxyl-3-methyl glutaryl coenzyme A (HMG-CoA) reductase, which catalyzes the rate-limiting step in cholesterol biosynthesis. The most important adverse effects of statins are increased concentrations of liver enzymes, myopathy, rhabdomyolysis and increased risk of diabetes.3, 4 In addition, reports of an increase in the potential risk of cognitive impairment with statins have been documented. 5
Teneligliptin is a dipeptidylpeptidase-4 inhibitor that inhibits the enzyme dipeptidyl peptidase-4 (DPP-4) and is a potent treatment option for type 2 diabetes (T2DM) either as monotherapy or in combination with other hypoglycemic agents. Gliptins are used for the treatment of T2DM when patients fail to show adequate glycemic control after dietary changes and exercise or with a combination of lifestyle modification and metformin /sulfonylurea treatment.6, 7They have relatively lesser adverse effects, weight neutral and do not cause any significant hypoglycemia.7,8Dyslipidemia is an important risk factor for cardiovascular complications in type 2 diabetic patients. It is unclear whether gliptins favorably improve lipid profile in type 2 diabetic patients as there are contradicting evidences from clinical studies. 9, 10
The DPP-4 inhibitors are also currently being evaluated for their effects on obesity and other metabolic traits.11, 12In addition, their role in chronic liver diseases including non-alcoholic steatohepatitis is also under investigation.13, 14
In animal studies, a beneficial effect on blood lipids has been detected in type 2 diabetic animal models like Zucker fatty rats. studies indicate a potential lipid lowering effect of teneligliptinin T2DM, the efficacy of the drug as an independent anti-hyperlipidemic agent is not yet determined.Thus the aim of this study was to evaluate the lipid lowering effect of teneligliptin in high fat diet rat model as there is paucity of studies assessing the hypolipidemic action of gliptins in diet induced hyperlipidemia model. So, in this study, we compared the effect of gliptins(teneligliptin) to that of statins (simvastatin) on hyperlipidemia induced in ratsusing high fat diet.
Materials and Methods
After the approval of the Institutional Animal Ethics Committee (Approval number:288/2015/IAEC),the study was done in the department of Pharmacology at a Medical college and research institute in Coimbatore. 22 healthy adult Sprague Dawley male rats of weight 180 g to 250g and age more than 2 months were chosen for the study. They were housed in polypropylene cages at normal ambient temperature and 12-hour light dark cycle. The animals were administered high cholesterol diet over a period of 4 weeks to induce hyperlipidemia after which,with 2 rats as controls, 10 rats were randomly allotted to each of the groups 1 and 2 following which they were administered the drug/vehicle during the last two weeks (5th,6th) along with normal standard chow diet.
Standard group (1): 10 rats given simvastatin(10mg/kg/day)
Treatment group (2): 10 rats given teneligliptin (20 mg/kg/day)
Control group (3): 2 rats given vehicle (sterile water)
The composition of high-lipid diet consisted of 1% (w/w) cholesterol, 10% (w/w) fat lard, 0.2% propylthiouracil, 5% yolk and 1% sodium tauroglycocholate.15The rats were allowed the high fat diet and water ad . The drugs simvastatin at a dose of 10 mg/kg and teneligliptin at a dose of 20 mg/kg were dissolved in sterile water and the control animals were given the vehicle (sterile water) all of which were administeredby oral gavage.
Blood samples (1.5 ml) were collected from tail vein of each rat using tail snip method under anesthesia. Blood sample collection was done at baseline, at the end of 4th week and 6th week. Lipid profiling was done to determine the levels of total cholesterol (TC), triglycerides (TG), low density lipoprotein cholesterol (LDL-C) and high density lipoprotein cholesterol (HDL-C). The LDL-C, HDL-C and (TC) estimations were done in auto-analyzer using GenX lipid parameter kits from Proton Biologicals and the (TG) was estimated in auto-analyzer using Coral clinical systems kit.
At the end of the study, the two control animals and three animals from the group 1 (standard) and group 2 (treatment) were sacrificed for collection of liver and aorta specimens for histopathology examination.Tissue sections of liver and aorta were preserved in 10% neutral buffered formalin later processed and stained using H&E stain.The slides were observed under microscope for fatty degeneration in liver and sub-intimal fat deposition in aorta.
Statistical Analysis
The changes in lipid parameters within the groupswere analyzed using paired t-test to detect statistically significant difference. One way ANOVAwith post-hocTukey test was done to evaluate the statistically significant difference between the treatment and standard groups. The results were expressed as mean ± SD. A value of p<0.05 was considered statistically significant. The histopathological changes were analyzed descriptively.
Results
All the three groups exhibited a rise in mean TG after induction which was statistically significant compared to the baseline. Following treatment with teneligliptin, the mean TG was 57.51 ± 16.00 mg/dl while that in the simvastatin group was 46.21 ± 16.05 mg/dl (Table 1) and the mean reduction of TG (28.70 ± 7.61 mg/dl) in group 2 was statistically different (p< 0.001) as that of the mean reduction in group 1(24.71 ± 18.11 mg/dl) with p = 0.021 (Table 2).However there was no statistical significance in the mean difference in TG levels between the two groups at the end of 6 weeks with p = 0.318(Table 3).
Likewise, serum TC level which increased significantly due to induction with high cholesterol diet demonstrated a reduction following treatment both in standard as wellas the treatment groups (Table 2). There was no statistically significant difference (p= 0.923) between the mean TC group 2 (122.22 ± 28.75 mg/dl) compared to that of group 1(127.21± 24.86 mg/dl) after treatment (Table 1 & 3).
Tables 1: Comparison of mean± standard deviation of the body weight and lipid parameters after treatment between the three groups
| Parameters | Group 1 | Group 2 | Group 3 |
| Body weight (g) | 267.62±18.91 | 258.37±37.20 | 324.33 ± 19.66 |
| Triglycerides(mg/dl) | 46.21±16.05 | 57.51±16.00 | 77.25 ± 10.08 |
| Total cholesterol(mg/dl) | 127.21 ± 24.86 | 122.21 ± 28.75 | 168.65 ± 22.58 |
| LDL(mg/dl) | 44.96 ± 7.61 | 40.70 ± 10.62 | 62.90 ± 4.49 |
| HDL(mg/dl) | 36.82 ± 6.61 | 33.08 ± 6.68 | 30.55 ± 3.34 |
In addition, at the end of 6 weeks, serum LDL-C also revealed a parallel reduction in both groups with the mean of group 1 (44.96±7.61 mg/dl) and 2(40.70 ± 10.62 mg/dl) at the end of intervention beingstatistically significant compared to that of mean serum after induction with p=0.002 & p=0.003 for groups 1 and 2 respectively using paired t test (Table 1 & 2). A statistical significance in mean LDL-C levels could not be detected (p = 0.735) between the two groups using ANOVA (Table 3). However, a similar trend was not evident for serum HDL-C. A fall in HDL-C level could be noted in both groups with induction compared to the baseline. The mean HDL-C value after induction for group 1 (33.65 ± 4.41 mg/dl)and group 2 (32.06 ± 4.27 mg/dl) revealed a rise with the standard (36.82 ± 6.61 mg/dl) as well as the treatment drug (33.08 ± 6.68 mg/dl) but the increase produced by either of the drugs was not statistically significantwith p=0.476 & p=0.796 for groups 1 and 2 respectively (Table 1 & 2).
Table 2: Comparison of lipid parameters within groups using paired t test
| Parameter (mg/dl) | Group | Pair | Mean Paired difference | Std. Deviation | Std. Error Mean | P value |
| TG | Group1 | Baseline
Induction |
-54.20 | 10.83 | 4.42 | .000 |
| Group1 | Induction
Treatment |
24.71 | 18.11 | 7.39 | .021 | |
| Group2 | Baseline
Induction |
-39.51 | 8.92 | 3.64 | .000 | |
| Group2 | Induction
Treatment |
28.70 | 7.61 | 3.10 | .000 | |
| TC | Group1 | Baseline
Induction |
-181.80 | 28.64 | 10.82 | .000 |
| Group1 | Induction
Treatment |
132.95 | 23.57 | 8.90 | .000 | |
| Group2 | Baseline
Induction |
-129.35 | 31.98 | 10.66 | .000 | |
| Group2 | Induction
Treatment |
66.85 | 35.02 | 11.67 | .000 | |
| LDL-C | Group1 | Baseline
Induction |
-35.03 | 8.51 | 3.47 | .000 |
| Group1 | Induction
Treatment |
21.06 | 8.82 | 3.60 | .002 | |
| Group2 | Baseline
Induction |
-31.30 | 6.79 | 2.77 | .000 | |
| Group2 | Induction
Treatment |
21.13 | 9.72 | 3.97 | .003 | |
| HDL-C | Group1 | Baseline
Induction |
11.88 | 5.49 | 2.24 | .003 |
| Group1 | Induction
Treatment |
-3.16 | 10.07 | 4.11 | .476 | |
| Group2 | Baseline
Induction |
14.05 | 5.21 | 2.12 | .001 | |
| Group2 | Induction
Treatment |
-1.01 | 9.11 | 3.72 | .796 |
Thus the net effect of teneligliptin evident was a statistically significant reduction in TC (p<0.001), TG (p<0.001) and LDL-C (p=0.003), but not the HDL-C (p=0.796) (Table 2 & Fig.1).A between group analysis using one way ANOVA revealed that there was no statistically significant difference between the groups treated with simvastatin and teneligliptin in all the four lipid parameters studied (Table 3).
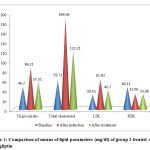 |
Figure 1: Comparison of means of lipid parameters (mg/dl) of group 2 |
Table 3: Comparison between groups using one way
| Parameters | Mean difference between group 1 and 2 | Standard error | Significance |
| Body weight(g) | 9.25 | 14.67 | .805 |
| Triglycerides(mg/dl) | 11.30 | 9.53 | .318 |
| Total cholesterol(mg/dl) | 4.99 | 13.11 | .923 |
| LDL(mg/dl) | 4.26 | 5.64 | .735 |
| HDL(mg/dl) | 3.73 | 3.32 | .516 |
Compared to baseline mean body weight of group 1(273.75±34.13 g) and group 2 (274.62±37.20 g), there was a rise in body weight in both standard (286.62±21.59 g) and treatment groups (280.87±40.99 g)after induction though the increase was not statistically significant. In addition, a reduction in body weight was discernable in the study animals of both groups with treatment using standard/drug, yet the fall in mean body weight was also not statistically significant in both groups. At the end of 6th week, the mean body weight of the standard group (267.62±18.91 g) did not differ significantly (p=0.805) from that of the treatment group(258.37± 37.20 g)(Table 1 & 3).
Histopathologic examination using H&E stain revealed a normal liver section in groups 1 and 2 while mild fatty changes in the form of intracytoplasmic vacuolation with occasional areas of congestion could be detected in the control group. This indicates therapy with both simvastatin and teneligliptin had reversed the mild fatty change induced by the disease model. Sections of aorta on histopathology examination failed to reveal any significant changes in all three groups including the control group indicating that induction for a period of 4 weeks withhigh fat diet had not caused any significant atherosclerotic changes in the aorta of our study animals (Fig.2).
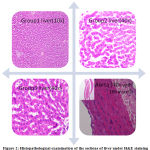 |
Figure 2: Histopathological examination of the sections of liver under H&E staining |
Fig.2 shows normal liver section in groups 1 and 2 while mild fatty changes in the form of intracytoplasmicvacuolation with occasional areas of congestion in the control group. Section of aorta of group3 reveals normal histology
Discussion
This study which was done in a rat model of diet induced hyperlipidemia has explicitly brought out the lipid lowering property of teneligliptin. This study has demonstrated that there is no statistical significance in the reduction in blood lipids between simvastatin and teneligliptin including serum TC, TG, LDL-C. In addition, though the change in mean serum HDL-C revealed a greater rise with simvastatin compared to teneligliptin, the rise was not statistically significant in both groups implying that the beneficial effect of teneligliptin on HDL-C levels closely paralleled that of simvastatin though had lesser magnitude to be statistically significant.
Teneligliptin, classified as a class III DPP-4 inhibitor, has a unique structural feature that provides strong binding to DPP-4 enzymes compared with other gliptins and additional pleiotropic benefits6.The mechanism of action of teneligliptin which is used as a hypoglycemic agent in the treatment of T2DM is to amplify the levels of incretins namely glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide in response to meal intake, in turn resulting in augmented insulin secretion and attenuated glucagon secretion.6,7
The hypolipidemic effect of teneligliptin is probably due to increased lipid mobilization from the adipose tissue and its oxidation by sympathetic activation facilitated through GLP-1 receptor activation, thereby reducing blood lipid levels as demonstrated by Boschmann et al.16
Also, the administration of teneligliptin has been shown to reduce the body weight in six-weeks-old C57BL/6N mice fed on a high-fat diet. The study has also established that teneligliptin increased oxygen consumption by 22% apart from proving that adipocyte hypertrophy and hepatic steatosis induced by a high-fat diet were suppressed by teneligliptin. In addition, a reduction in mean adipocyte size and hepatic TG content were demonstrable in this study.12
Another study investigating the effectiveness of teneligliptin in a mouse model of non-alcoholic steatohepatitis revealed that DPP4 inhibitors, by amplifying GLP-1 induced AMPK activation in hepatocytes inhibits hepatic lipogenesis, thus consequentially inhibiting cholesterol and TG biosynthesis. The AMPK activation also promotes fatty acid β-oxidation and reduction of free fatty acid.14Thus it is obvious that multiple pathophysiological mechanisms are potentially contributory to the hypolipidemic effect of teneligliptin.
However two clinical studies in Japanese population have yielded conflicting results on teneligliptin’s effect on lipid profile. But both these studies were done in a small sample size which could be one of the reasons in discordance in their results.9, 10 Yet another large clinical study done in a Korean population wherein about 1732 patients on any of the six other gliptins including alogliptin, gemigliptin, sitagliptin, vildagliptin, saxagliptin and linagliptin were switched over to teneligptin, the effects of which were recorded at the end of 12 weeks.This study demonstrated that the weight decreased significantly by a mean of 0.4 kg from baseline in addition to a statistically significantdecrease in the mean BMI by 0.1 kg/m2. Among the serum lipid parameters, TC and LDL-C levels decreased from baseline to week 12 (p < 0.05). Though a favorable trend in the change from mean baseline values were noted for TG and HDL-C they were not statistically significant.17
The greater potency and additional beneficial effects of teneligliptin could be attributed to the fact that it binds more tightly to the DPP-4 enzyme compared to other gliptins because of the “J-shaped” structure formed by five rings.18 Thus the long-duration and strong binding property of teneligliptin could potentially yield greater clinically meaningful metabolic benefits.
Conclusion
This study has delineated the hypolipidemic activity of teneligliptin which will be of potential clinical value in the therapeutic use of teneligliptinin type 2 diabetic patients especially in the face of increased concomitance in the occurrence of T2DM and hyperlipidemia.
Conflicts of Interest
No conflicts of interest.
Funding Source
The study was funded by an Institutional intramural grant, “the PRIME grant” for academic projects by faculty investigators.
References
- Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics — 2018 update: a report from the American Heart Association. Circulation. 137(12):e67-e492 (2018).
CrossRef - Joshi SR, Anjana RM, Deepa M, Pradeepa R, Bhansali A, Dhandania VK et al. Prevalence of dyslipidemia in urban and rural India: the ICMR-INDIAB study. PLoS One.9(5):e96808 (2014).
CrossRef - Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins: a study-level network meta-analysis of 246 955 participants from 135 randomized, controlled trials. Circulation: Cardiovascular Quality and Outcomes. 6(4):390-9 (2013).
CrossRef - Newman CB, Preiss D, Tobert JA, Jacobson TA, Page RL, Goldstein LB, Chin C, Tannock LR, Miller M, Raghuveer G, Duell PB. Statin safety and associated adverse events: a scientific statement from the American Heart Association. Arteriosclerosis, thrombosis, and vascular biology. 39(2):e38-81 (2019).
CrossRef - Schultz BG, Patten DK, Berlau DJ. The role of statins in both cognitive impairment and protection against dementia: a tale of two mechanisms. Translational neurodegeneration. 7(1):5 (2018).
CrossRef - Sharma SK, Panneerselvam A, Singh KP, Parmar G, Gadge P, Swami OC. Teneligliptin in management of type 2 diabetes mellitus. Diabetes, metabolic syndrome and obesity: targets and therapy.9:251-260(2016).
CrossRef - Kishimoto M. Teneligliptin: a DPP-4 inhibitor for the treatment of type 2 diabetes. Diabetes, metabolic syndrome and obesity: targets and therapy.6:187-195 (2013).
CrossRef - Singh AK. Efficacy and safety of teneligliptin. Indian journal of endocrinology and metabolism. 21(1):11-17(2017).
CrossRef - Kutoh E, Hirate M, Ikeno Y. Teneligliptin as an initial therapy for newly diagnosed, drug naive subjects with type 2 diabetes. Journal of clinical medicine research. 6(4):287-94 (2014).
CrossRef - Kusunoki M, Sato D, Nakamura T, Oshida Y, Tsutsui H, Natsume Y, Tsutsumi K, Miyata T. DPP-4 inhibitor teneligliptin improves insulin resistance and serum lipid profile in Japanese patients with type 2 diabetes. Drug research. 65(10):532-4 (2015).
CrossRef - Martin KA, Mani MV, Mani A. New targets to treat obesity and the metabolic syndrome. European journal of pharmacology.763:64-74 (2015).
CrossRef - Fukuda-Tsuru S, Kakimoto T, Utsumi H, Kiuchi S, Ishii S. The novel dipeptidyl peptidase-4 inhibitor teneligliptin prevents high-fat diet-induced obesity accompanied with increased energy expenditure in mice. European journal of pharmacology. 723:207-15 (2014).
CrossRef - Itou M, Kawaguchi T, Taniguchi E, Sata M. Dipeptidyl peptidase-4: a key player in chronic liver disease. World journal of gastroenterology. 19(15):2298-2306 (2013).
CrossRef - Ideta T, Shirakami Y, Miyazaki T, Kochi T, Sakai H, Moriwaki H, Shimizu M. The dipeptidyl peptidase-4 inhibitor teneligliptin attenuates hepatic lipogenesis via AMPK activation in non-alcoholic fatty liver disease model mice. International journal of molecular sciences. 16(12):29207-18 (2015).
CrossRef - Zhang Q, Wang GJ, Ji-ye A, Wu D, Zhu LL, Ma B, Du Y. Application of GC/MS-based metabonomic profiling in studying the lipid-regulating effects of Ginkgo biloba extract on diet-induced hyperlipidemia in rats. ActaPharmacologicaSinica. 30(12):1674-87 (2009).
CrossRef - Boschmann M, Engeli S, Dobberstein K, Budziarek P, Strauss A, Boehnke J, Sweep FC, Luft FC, He Y, Foley JE, Jordan J. Dipeptidyl-peptidase-IV inhibition augments postprandial lipid mobilization and oxidation in type 2 diabetic patients. The Journal of Clinical Endocrinology & Metabolism. 94(3): 846-52 (2009).
CrossRef - Kim HJ, Kim YS, Lee CB, Choi MG, Chang HJ, Kim SK, Yu JM, Kim TH, Lee JH, Ahn KJ, Kim K. Efficacy and Safety of Switching to Teneligliptin in Patients with Type 2 Diabetes Inadequately Controlled with Dipeptidyl Peptidase-4 Inhibitors: A 12-Week Interim Report. Diabetes Therapy. 10(4): 1271-1282 (2019).
CrossRef - Nabeno M, Akahoshi F, Kishida H, Miyaguchi I, Tanaka Y, Ishii S, et al. A comparative study of the binding modes of recently launched dipeptidyl peptidase IV inhibitors in the active site. BiochemBiophys Res Commun. 434(2):191–196 (2013).
CrossRef








