Juala Catherine Jebaraj* and Bhuvaneswari B
Department of Periodontology, Tagore Dental College and Hospital Chennai, India 600 127
Corresponding Author E-mail : catherinej88@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1870
Abstract
Regenerative medicine aims at replacing lost or defective tissues with new functioning ones. Regenerative medicine uses stem cells which are found in every organ of the body and provides a potential area for research. Pancreatic stem cells have received broad attention as it can be used in the treatment of irreparable disease of the pancreas such as Type I Diabetes Mellitus and pancreatic ductal adenocarcinoma. This review gives a brief insight into the development of pancreatic stem cells, isolation techniques, therapeutic applications and the challenges faced in using adult pancreatic stem cells.
Keywords
Adult Pancreatic Stem Cells; Pancreatic Ductal Adenocarcinoma; Stem Cells; Type I Diabetes Mellitus.
Download this article as:| Copy the following to cite this article: Jebaraj J. C, Bhuvaneswari B. Human Pancreatic Adult Stem Cells – Is It A Gleam of Hope to Patients with Type I Diabetes Mellitus? Biomed Pharmacol J 2020;13(1). |
| Copy the following to cite this URL: Jebaraj J. C, Bhuvaneswari B. Human Pancreatic Adult Stem Cells – Is It A Gleam of Hope to Patients with Type I Diabetes Mellitus? Biomed Pharmacol J 2020;13(1). Available from: https://bit.ly/2S56pLz |
Introduction
For several decades researchers have been trying to understand the body’s ability to repair and replace the cells and tissues of some organs. After many years of work pursuing on cell repair mechanisms, scientists have now focused their attention on adult stem cells. It has long been known that stem cells are capable of renewing themselves and that they can generate multiple cell types. Today, there is adequateevidence that stem cells are present in far more tissues and organs than it was once thought. These cells are capable of developing into tissues and organs. Efforts are now underway to exploit this new found capability of the stem cells with the goal of devising new and more effective treatments for many diseases.
Adult stem cells, like all stem cells, share at least two characteristics. First, they have long-term self-renewal. Second, they can give rise to mature cell types that have characteristic morphologies and specialized functions.(1) Typically, stem cells generate an intermediate cell type or types before they reach their fully differentiated state. The intermediate cell is called a precursor or progenitor cell.(2) Progenitor or precursor cells in fetal or adult tissues are partly differentiated cells that divide and they give rise to fully differentiated/mature cells.
Among the various adult stem cells currently being studied, pancreatic adult stem cells have gained much attention because of it can be potentially used in the treatment of Type I Diabetes Mellitus (Type I DM).
Background/History
Development of Pancreas and Islets of Langerhans
During embryogenesis, the pancreas develop from the notochord which invaginates to form the dorsal and ventral primodia (pancreatic buds). The pancreatic buds are formed from the foregut by repression of Sonic Hedgehog (Shh) gene and are destined to become the pancreas. Active Notch signaling promotes the development of the exocrine portion of the pancreas whereas inactive Notch signaling promotes development of the endocrine portion of the pancreas. The acinar and ductal epithelium comprise the exocrine portion and the Islet cells form the endocrine portion.
Evidence from recent studies have unveiled the master switch genes that encode for the transciption factors that regulate differentiation of the cells in the pancreas. Both the exocrine and the endocrine cells of the pancreas develop from a common pool of endodermal progenitor cells. The major gene in differentiation is Pdx-1 gene which is a family of homeobox containing genes. Pdx-1 is expressed by all epithelial cells is early stages of development and in later stages it is downregulated by all cells except the β cells and the delta cells of the islets. Thus Pdx-1 gene acts as a master switch for development of the endocrine cell of the pancreas. The other major gene is Ptf-1 which regulates the development of the exocrine cells of the pancreas. Progenitors of the islet cells also express Neurog 3/ Ngn 3.(3)
The following flowchart illustrates the important events in the embryogenesis of the pancreas
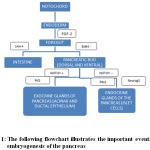 |
Figure 1: The following flowchart
|
Evidence for Adult Stem Cells in Pancreas
The pancreas being a source of stem cells was long debated since pancreas is an organ made up of well differentiated exocrine and endocrine cells. However several in vitro experimental studies have confirmed the fact that pancreas are a source of adult stem cell.
Breljeet al in an invitro study has shown that conditions such as obesity and gestational diabetes cause reversible increase in β cell mass in mice and human islets. Furthermore the β cell mass returns to normal when systemic control of diabetes and obesity isachieved. (4)
Immunohistochemicalanalysis by Bouwensshows that in nearly 15% of all β cells were smaller and immature indicating the presence of immature progenitor cells in the islets that become differentiated into mature cells when it is required. (5)
Stem Cell Niche in Adult Pancreas
Where is the Adult Stem Cells Located in the Pancreas?
There are two theories that have been currently proposed regarding the stem cell niche in adult pancreas.(6)
The first theory is the ‘Neogenesis’ theory where researchers believe that the β-islet cells arise from non islet stem cells.
The second theory is the ‘Expansion’ theory where the β cell mass expands from the existing β cells.
Most investigators suggest the presence of adult stem cells in the pancreatic ducts based on several experimental models.
Model 1
Chemical ablation or destruction of the β cells can be achieved by the use of cytotoxic drugs such as Streptozotocin or Alloxan. In an experiment conducted on mice by Fernades et al(7)the regeneration of the β cells after they have been selectively ablated by Streptozotocin was assessed. It was proved that the islet cells contain progenitor cells that can replace the β cells that has been destroyed by Streptozotocin or allantoin. These cells are called as Islet Progenitor cells (IPC).
Similar results were shown in a study by Dor et al where terminally differentiated β cells retained its proliferative capacity indefinitely(8).
Model 2
Another pancreas regeneration model by Bonner- Weir which consisted of surgical removal of the pancreas by pancreatectomy. Subtotal pancreatectomy (90%) is followed by rapid proliferation of the ductal cells to form acini. islet cells and the ductal cells. Based on this model has been suggested that the stem cells in pancreas are located in the ductal epithelium. (9)
Model 3
Another experimental model by Wangconsists of complete obstruction of the exocrine pancreas by ligation of the ducts. A ligature is placed which blocks the exocrine secretion from being evacuated. In the ligated areas of the pancreas significantremodelling of the ductal epithelium occurs which has been found to occur through the transdifferentiation of the acinar cells into the ductal cells. The above mechanism is termed as acinoductal metaplasia. There was also a significant increase in the β cell mass after ligation, Since the β cells have limited mitotic activity, it has been proposed that the new β cells originated from the ductal complex since these new β cells express CK 20 a ductal marker. (10)
In a similarrecent study by Xu. X et al showed by genetic tracing Nng-3 expressing cells arising from the ductal epithelium in ligature placed rats.(11)
Recent studies by Thorel et al have suggested the occurrence of interconversion among endocrine cells, especially between α and β cells. Under extremely severe conditions leading to almost total β-cell loss, transdifferentiation of α-cells into β-cells occur.(12)
Plasticity of Pancreatic Stem Cells
Rodent experimental studies have shown bi-potential stem cells of the pancreas wherein precursor cells from the ductal epithelium also form hepatocytes suggesting a common hepatopancratic stem cell. Since liver and pancreas arise from the same bi-potential precursors in the anterior endoderm, it is reasonable to speculate that two closely related tissues may be inter-convertible. Specifically, it was believed that cells with properties similar to hepatic oval cells (a proposed hepatic progenitor population) also reside in the pancreas, which could transdifferentiate into functional hepatocytes upon transplantation. (13)
Isolation of Adult Stem Cells
Generating Islet progenitor cells from Embryonic stem cells or genetically reprogramming embryonic cells to function as β islet cells still continues to remain a challenge. Howevera number of procedures have been suggested for the isolation of adult stem cells from the pancreas. The basic protocol was given by Peck et al in 1995 .(14)
Invitro Culture Steps:
There are four steps in the protocol to isolate adult pancreatic stem cells as given by Peck.
Step 1
Isolation of pancreatic duct cells from partially digested pancreas and grown in culture using Eagle’s hank’s Amino Acids (EHAA) medium also called as Clicks medium, to enrich the epithelial cell population.
Step 2
Addition of serum from diabetic individual to favor the budding of Induced Pluripotent Stem cells (IPC) from the epithelial monolayer. These IPC further proliferate into spheroid structures.
Step 3
The cells of the spheroid structure are stimulated by high concentration glucose to promote proliferation and differentiation of β cells.
Step 4
Implantation into an in vivo environment to complete maturation of the β cells.
Functional Efficacy of Invitro Generated Islets
The functional efficacy of the invitro generated stem cells has been analyzed by a implantation experiments in diabetic mice by Ramiya et al.(15)
In the first experiment 300 IPSC derived islets were implanted in female diabetic insulin dependent mice. After implantation of the IPSC derived islets the mice were weaned of insulin. Within one week of implantation the mice showed stable blood glucose levels (180-220 mg/dl). The mice in the control group which did not receive IPSC derived islets showed severe wasting disease.
In the second experiment 1000 IPSC derived islets were implanted subcutaneously in diabetic mice and these mice showed more stable blood glucose level (100-150mg/dl).
The above two experimental studies show that invitro generated islet of Langerhans (β cells) 0effectively control blood glucose level and help maintain normoglycemic blood level.
Advantages of Stem Cell Derived Islets
Isolation procedure is relatively simple. They can be isolated and delivered by percutaneous catheterization of the portal vein under local anaesthesia.
The cultured islets can be genetically modified in vitro to resist any undesired immune response.(16)
Disadvantages of Stem Cell Derived Islets
Obtaining adequate number of islets through in vitro culture is quite challenging and require stringent protocol to be followed.
Patient is required to use systemic immunosuppressive agents throughout life in order to prevent untoward immune response against the implanted islets. Immunosupressive agents are required even when autologous stem cells are used.(17)
Applications
Type I Diabetes Mellitus
In individuals genetically predisposed to Type I Diabetes mellitus (Type I DM), the organization and function of the β cell clusters are affected by autoimmune response. Since β cells have limited regenerative capacity the cells destroyed by the body’s own immune response is not replaced. In such patients the pancreatic islets contain only non β cells. The result is that such patients require lifelong insulin therapy to maintain normoglycemic levels. While insulin remains to be the only treatment option, only ecto pancreatic transplants or islet implants represent a cure for patients with Type I Diabetes Mellitus.
Unfortunately availability of donor for transplant of pancreas or as a source of islets for implantation is acutely limited. Furthermore the transplanted organ or islets is allogenic and the patient is required to take lifelong immunosupressive therapy.
Use of islet cells generated invitro from autologous pancreatic stem cells can represent a major breakthrough in the treatment of Type I Diabetes Mellitus.(18)
Pancreatic Ductal Adenocarcinoma
Pancreatic cancer is one of the deadliest cancers and has been reported to be the leading cause of cancer death in the United States. Pancreatic cancer is highly resistant to therapy. It has been recently identified that the reason for the resistant to treatment is due to the pancreatic cancer stem cells (CSC). The CSC are responsible for tumor initiation, growth, metastasis and resistant to treatment. Identifying the signaling pathway of these CSC and inactivating them therapeutically would propose a possible cure for pancreatic cancer and improve the survival rate of patients affected by this life threatening deadly disease. (19)
Challenges
Although stem cells have been isolated from ductal epithelium of both healthy and diabetes patient and successfully cultured, expanded and differentiated into β islet insulin producing cells, there are a few roadblocks which makes stem cell therapy in Type I DM patients challenging.
The factors which control maturation of the immature islet cells in an invivo environment is not yet fully understood. Only if it is fully understood Islet transplant can be carried out effectively.
The number of IPSC derived Islets to achieve diabetic control is difficult to obtain from autologous ductal cells.
Cell to cell contact and cell communications are essential for beta cells to produce insulin. Simulating this microenvironment in an invitro environment is difficult.
Discussion
Type I Diabetes Mellitus is increasing globally and around1 million cases are reported in India. An intrinsic disease like Diabetes Mellitus is not entirely preventable; can have long lasting systemic complications which are often debilitating, irreversible and can severely affect the quality of life also. The present lifestyle compromises of individuals resulting in stress and obesity also increases risk of onset of the disease. What is more exasperating is the fact that these patients need lifelong insulin therapy to maintain normal blood glucose level. Β- cells cultured from adult pancreatic stem cell offer a ray of hope to patients suffering from Type-I diabetes mellitus and life- threatening conditions like pancreatic adenocarcinoma. Although isolation and culture of islet cells is undemanding the most challenging problem is to maintain the functionality of the islet cells invivo and the life long need to take immunosupressants to avoid immune rejection.
Future Directions
Identifying the genes that are involved in the development of the islets may provide necessary information to stimulate the end stage development of mature β cells of the islets of Langerhans invitro so that β islet cell transplant can be effectively used in the management of Type I Diabetes Mellitus.
Conclusion
What lies ahead for the use of pancreatic adult stem cells is unknown, but it is certain that there are many research questions to be answered and that these answers hold great promise for the future. Expanding research in the field of adult pancreatic stem cell in humans could result in ground breaking therapeutic intervention in the treatment of Type I diabetes mellitus and bring hope to the patients who are affected by this disease to lead a normal life.
References
- Burke ZD, Thowfeequ S, Peran M, Tosh D. Stem cells in the adult pancreas and liver. Biochem J. 2007;404(2):169–178.
CrossRef - Lin HT, Chiou SH, Kao CL, et al. Characterization of pancreatic stem cells derived from adult human pancreas ducts by fluorescence activated cell sorting. World J Gastroenterol. 2006;12(28):4529–4535.
CrossRef - Edlund H. Transcribing pancreas. Diabetes. 1998;47(12):1817-1823.
CrossRef - Brelje T. Effect of homologous placental lactogens, prolactins, and growth hormones on islet B-cell division and insulin secretion in rat, mouse, and human islets: implication for placental lactogen regulation of islet function during pregnancy. Endocrinology. 1993;132(2):879-887.
CrossRef - Bouwens L, Pipeleers D. Extra-insular beta cells associated with ductules are frequent in adult human pancreas. Diabetologia. 1998;41(6):629-633.
CrossRef - Murtaugh LC, Kopinke D. Pancreatic stem cells. 2008 Jul 11. In: StemBook Cambridge (MA): Harvard Stem Cell Institute; 2008-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK27059
CrossRef - Fernandes A. Differentiation of New Insulin-Producing Cells Is Induced by Injury in Adult Pancreatic Islets. Endocrinology. 1997;138(4):1750-1762.
CrossRef - Dor Y, Brown J, Martinez O, Melton D. Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429(6987):41-46.
CrossRef - Bonner-Weir S, Baxter L, Schuppin G, Smith F. A second pathway for regeneration of adult exocrine and endocrine pancreas. A possible recapitulation of embryonic development. Diabetes. 1993;42(12):1715-1720.
CrossRef - Wang R, Kluppel G, Bouwens L. Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia. 1995;38(12):1405-1411.
CrossRef - Xu X, D’Hoker J, Stangé G, Bonné S, De Leu N, Xiao X. β Cells Can Be Generated from Endogenous Progenitors in Injured Adult Mouse Pancreas. Cell. 2008;132(2):197-207.
CrossRef - Thorel F, Népote V, Avril I, Kohno K, Desgraz R, Chera S. Conversion of adult pancreatic α-cells to β-cells after extreme β-cell loss. Nature. 2010;464(7292):1149-1154.
CrossRef - Yi F, Liu G, Belmonte J. Rejuvenating liver and pancreas through cell transdifferentiation. Cell Research. 2012;22(4):616-619.
CrossRef - Peck A.B and Cornelius J.G . Invitro growth of mature pancreatic Islet of langerhans from single, pluripotent stem cell isolated from prediabetic adult pancreas. Diabetes. 1995;44(10 A).
- Ramiya V, Maraist M, Arfors K, Schatz D, Peck A, Cornelius J. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nature Medicine. 2000;6(3):278-282.
CrossRef - Rita Bottino, Suzanne Bertera, Maria Grupillo, Patricia R. Melvin, Abhinav Humar,George Mazariegos et al. Isolation of Human Islets for Autologous Islet Transplantation in Children and Adolescents with Chronic Pancreatitis. Journal of Transplantation 2012:1-8.
CrossRef - Julie B. Sneddon, Qizhi Tang, Peter Stock, Jeffrey A. Bluestone, Shuvo Roy, Tejal Desai and Matthias Hebrok Stem Cell Therapies for treating Diabetes: Progress and Remaining Challenges. Perspective 2018; 22(6): 810-823.
CrossRef - Figliuzzi M, Bonandrini B, Silvani S, Remuzzi A. Mesenchymal stem cells help pancreatic islet transplantation to control type 1 diabetes. World J Stem Cells. 2014;6(2):163–172.
CrossRef - Rao C. New insights into pancreatic cancer stem cells. World Journal of Stem Cells. 2015;7(3):547.
CrossRef







