Sahar Ezeldien , Waleed F Khalil
, Waleed F Khalil , Mostafa Fayez
, Mostafa Fayez and Mohamed M. Abdel-Daim
and Mohamed M. Abdel-Daim
Pharmacology Department, Faculty of Veterinary Medicine, Suez Canal University, Ismailia 41522, Egypt
Corresponding Authro E-mail: abdeldaim.m@vet.suez.edu.eg
DOI : https://dx.doi.org/10.13005/bpj/1792
Abstract
Doxorubicin is one of the most effective anthracycline anticancer drugs, but it causes several adverse effects. Our study was designed to assess the consequences of combining doxorubicin with chloroquine or gemifloxacin. Drugs cytotoxicity was assessed on two different cell lines; A549 lung adenocarcinoma and MCF7 breast cancer. The in-vitro oxidative stress was also measured. In the in-vivo experiment, Ehrlich ascetis carcinoma-bearing mice, different treatments with doxorubicin, chloroquine, gemifloxacin and their combinations were evaluated. Survival indices (MST and ILS%) and blood biochemical parameters as well as the histopathological picture were studied. Results showed that, doxorubicin combinations were more cytotoxic on MCF7 and A549 cell lines than doxorubicin alone. The combinations significantly decreased the oxidative stress resulted from doxorubicin treatment. Furthermore, these combinations improved hematological parameters and histopathological pictures in the treated mice. In conclusion, chloroquine and gemifloxacin significantly enhance the antitumor properties of doxorubicin and reduce its toxicity.
Keywords
Cancer; Doxorubicin; Chloroquine; Gemifloxacin; Ehrlich
Download this article as:| Copy the following to cite this article: Ezeldien S, Khalil W. F, Fayez M, Abdel-Daim M. M. Chloroquine and Gemifloxacin Potentiate the Anticancer Effect of Doxorubicin: In-Vitro and In-Vivo Models. Biomed Pharmacol J 2019;12(4). |
| Copy the following to cite this URL: Ezeldien S, Khalil W. F, Fayez M, Abdel-Daim M. M. Chloroquine and Gemifloxacin Potentiate the Anticancer Effect of Doxorubicin: In-Vitro and In-Vivo Models. Biomed Pharmacol J 2019;12(4). Available from: https://bit.ly/2Q5RtvA |
Introduction
Cancer is a disease characterized by the uncontrolled division of abnormal cells of any tissue capable of spreading to other sites within the body. Mutation of the genetic apparatus of cells is the universally accepted basis of the pathogenesis of cancer (1). Most of the available antitumor chemotherapy still suffers from two problems; firstly, they are non selective for cancer cells, producing several side effects. The second is the drug resistance acquired by cancer cells (2). Currently, there is a growing interest in combining anticancer drugs to maximize efficacy and minimize systemic toxicity through lowering the drug doses. Doxorubicin (DOX) is one of the most effective anthracycline anticancer drugs, acting by inhibition of topoisomerase II (Topo II) enzyme producing multiple DNA breaks. Recently, its use is limited as it cause cardiotoxicity, bone marrow suppression and nephrotoxicity (3).
Quinolones, including gemifloxacin (GMF), are very essential antibacterial agents that are broadly used for curing of infections in human and animals (4). Other than the antibacterial activities of quinolones, certain members of this family show inhibition of eukaryotic Topo II enzymes in cultured mammalian cells and in-vivo tumor models (5, 6).
Chloroquine (CQ) and related antimalarial drugs inhibit drug efflux from the cell. They help in sensitizing resistant cells to the effect of anticancer drugs by increasing the intracellular concentrations of the anticancer agent (7). CQ inhibits the function of some membrane-associated proteins and multidrug resistance (MDR) protein families which support developing of anticancer resistance in several cancer types (8).
Therefore, our aim was to study the possible anticancer effect, as well as the adverse effect of both chloroquine and gemifloxacin individually and in combination with doxorubicin using cultured and animal models.
Materials and methods
Doxorubicin (Adriablastina 50mg®), chloroquine (Alexoquine 250mg®) and gemifloxacin (Floxguard 320mg®) were purchased from local pharmacy, Ismailia, Egypt. From the National Research Center, Cairo, Egypt; breast cancer (MCF7) and lung adenocarcinoma (A549) cell lines were obtained. Cell lines were cultured in RPMI medium with 10% fetal bovine serum, 2% penicillin, and 1% of amphotericin-B at 37 °C in humidified 5% CO2 atmosphere.
For the in-vivo study, Ehrlich ascetis carcinoma (EAC) bearing mice were obtained from the National Cancer Institute, Cairo, Egypt. EAC cells were transplanted in female mice through serial intraperitoneal (i.p.) injection of 1×106 viable tumor cells/mouse.
Antioxidant kits produced by Bio-diagnostic, Cairo, Egypt were used for assaying reduced glutathione (GSH), superoxide dismutase (SOD) and malondialdehyde (MDA) levels in MCF7 cells. Serum biochemical ready-made kits produced by Diamond Co., (Cairo, Egypt) were used for assaying urea, creatinine, alanine transaminase (ALT) and aspartate transaminase (AST) levels.
In the in-vitro study, the cytotoxic assay on MCF7 cells was measured using sulphorhodamine-B stain (SRB) method (9). Cytotoxic potential of DOX, CQ, GMF and their combinations on A549 cell line was determined by the MTT test (10). The in-vitro oxidative stress in the MCF7 cells treated with DOX, CQ, GMF, DOX plus CQ combination and DOX plus GMF combination (using half dose of each drug) was assessed as described by Salama and his colleagues (11) through measuring the levels of MDA, SOD and GSH.
In the in-vivo study, seventy female Swiss albino mice (10 mice/group) were housed in plastic cages (5 mice from each group were used for sample collections and the other 5 were kept for determining the survival parameters). At random, mice were divided into 7 groups. Group 1: normal control, mice were injected i.p. with saline (0.9% NaCl). Group 2: EAC control, mice didn’t receive any treatment. Group 3: DOX treated group, where mice were injected intravenously (i.v.) with DOX (2 mg/Kg BW) once weekly (12, 13). Group 4: CQ treated group, in which mice were orally treated with 25 mg CQ/Kg BW/per day (14). Group 5: GMF treated group, mice were treated orally with 25 mg GMF/Kg BW/day (15). Group 6: in which, mice received CQ in combination with DOX in the same previous doses. Group 7: where the mice were treated with GMF in combination with DOX as previously mentioned.
Survival study
The increased life span percentage (ILS%) and the mean survival time (MST) were calculated as indicated by the following equations:
ILS% = (T-C)/C×100, where T represents MST of treated animals and C represents MST of the EAC group.
MST = (day of first death + day of last death)/2.
ILS exceeding 25 % was considered as a significant anticancer effect (16). The intra-peritoneal ascetic fluid of tumor-injected mice was collected to determine tumor volume and tumor cell count.
Biochemical and histopathological studies
Blood samples were freshly collected in EDTA tubes for assaying of hemoglobin level, RBCs, WBCs and differential leukocytic counts. Another portion of blood samples were left for clotting, centrifuged at 2000ÍG for 10 min to separate serum for the biochemical assay of ALT, AST, urea and creatinine. Specimens of the heart, liver and kidney were kept in buffered formalin (10%) to examine the histopathological changes.
Result
In-vitro experiment
Our result showed that, the combinations were more effective than DOX alone on both MCF7 and A549 lines. IC50 of DOX, CQ, GMF and their combinations on MCF7 and A549 cell lines were shown in table 1.
Table 1: In-vitro cytotoxic effect of DOX, CQ, GMF and their combinations on MCF7 breast cancer and A549 lung cancer cell lines.
| IC50 (µg/ml) | Drug | |
| A549 cells | MCF7 cells | |
| 0.45 | 15.1 | Doxorubicin |
| 0.13 | 47.8 | Chloroquine |
| 17.0
|
17.1 | Gemifloxacin |
| 0.3 | 5.2 | DOX plus CQ |
| 0.3 | 9.1 | DOX plus GMF |
IC50 is the drug concentration causing 50% inhibition of the cell viability.
Regarding to the oxidative stress, DOX, GMF and CQ significantly decreased SOD and non-significantly decreased GSH levels when compared to MCF7 control group. The combinations induced less oxidative stress than DOX alone (table 2).
Table 2: In-vitro assessment of oxidative stress induced by DOX, CQ, GMF and their combinations.
| Treatment | SOD
U/ml |
GSH
mmol/L |
MDA
nmol/ml |
| MCF7 control | 0.94±0.09 | 0.12±0.006 | 11.1±1.3 |
| Doxorubicin | 0.19±0.06** | 0.03±0.001 | 24.1±4.1* |
| Chloroquine | 0.27±0.04** | 0.04±0.005 | 16.5±1.0 |
| Gemifloxacin | 0.46±0.05* | 0.06±0.001 | 13.6±0.4 |
| DOX plus CQ | 0.73±0.08# | 0.87±0.040** # | 11.5±2.7# |
| DOX plus GMF | 0.61±0.06# | 0.68±0.050** # | 13.1±0.6 |
Data are represented as Mean± SE.
(*) represent a significant variation between MCF7 control group and other treated groups.
(#) represent a significant variation between DOX treated group and other treated groups.
In-vivo experiment
From the survival data point of view, DOX, as a mono therapy, did not show the expected anticancer effect while its adverse effects were observed. Doxorubicin treatment decreased MST when compared to EAC control group. Moreover, CQ, GMF and their combinations with DOX significantly increased ILS% when compared to that of DOX treated group. Effects of DOX, CQ, GMF and their combinations on MST, ILS%, tumor volume and tumor cell count were shown in table 3.
Table 3: Effect of DOX, CQ, GMF and their combinations on MST, ILS%, tumor cell count and tumor volume in EAC bearing mice.
| Treatment | MST (day) | ILS% | Tumor cell count
CellÍ106/ml |
Tumor volume (ml) |
| Normal control | – | – | 00±00 | 00±00 |
| EAC control | 15.5 | – | 30.0±3.2 | 10.0±1.00 |
| Doxorubicin | 14.5 | -6.40 | 22.0±2.2 | 7.9±0.10 |
| Chloroquine | 17.5# | 12.90# | 27.0±1.3 | 8.5±0.12 |
| Gemifloxacin | 16.5 | 6.44# | 28.5±0.5 | 7.9±0.15 |
| DOX plus CQ | 16.0 | 3.22# | 21.0±1.3 | 8.0±0.15 |
| DOX plus GMF | 15.5 | 0.00# | 19.0±1.2* | 7.8±0.31 |
Data are represented as Mean± SE (5animals/group).
(*) represent a significant difference between EAC control group and other treated groups.
(#) represent a significant difference between DOX treated group and other treated groups.
EAC control, CQ and GMF treated groups showed significant increase in RBCs, Hb, HCT, WBCs and neutrophils count when compared to those of normal control. DOX treated mice showed significant decrease in RBCs, Hb, HCT and WBCs count when compared to those of EAC control group. The combination of DOX plus CQ significantly increased RBCs, WBCs and neutrophils count when compared to DOX treated group. Effect of drugs on hematological parameters was shown in tables 4.
Table 4: Effect of DOX, CQ, GMF and their combinations on hematological parameters.
| Treatment | RBCs
Cell Í 106/µl |
Hb
g/dl |
HCT% | WBCs
Cell Í 103/µl |
Segmented Neutrophils% | Band Neutrophils% | Lymphocytes% | Monocytes% | Eisinophils% |
| Normal control | 5.0±0.63 | 12.2±0.8 | 35.7±3.5 | 7.9±0.1 | 12.6±1.5 | 1.6±0.66 | 78.7±2.6 | 4±1.52 | 1±0 |
| EAC control | 7.6±0.08 | 17.2±0.8 | 54±0.9 | 34.5±2.7 | 51.6±4.4 | 4.6±0.88 | 59±6.1 | 7±0.57 | 1±0 |
| Doxorubicin | 4.8±0.05** | 12±1.03** | 36±3** | 8.7±1.3** | 33.3±6 | 2.3±0.66 | 53.6±4.2 | 9.6±0.66 | 2.3±0.3 |
| Chloroquine | 7.2±0.05# | 18±0.5# | 51.3±4.6# | 25.2±1.3** # | 60.6±6.2# | 4±1.15 | 27±1.2* | 12.3±0.88* | 2±0 |
| Gemifloxacin | 7.1±0.05# | 14.43±0.8 | 45.6±1.7 | 24.9±1.1** # | 59.3±6.3# | 6.6±0.88# | 25±2.9* # | 11±0.57 | 2.3±0.9 |
| DOX plus CQ | 7±0.07# | 14.1±0.1 | 44.5±2.2 | 17.3±1.4** # | 63.3±3.3# | 7.3±0.33# | 22±4.2** # | 7.3±1.85 | 1.6±0.3 |
| DOX plus GMF | 4.5±0.06** | 12.2±0.5** | 34.5±0.9** | 12.3±1.0** | 70±5.7# | 6.3±0.88# | 53.3±12 | 6±0.57 | 1±0 |
Data are represented as Mean± SE (5animals/group).
(*) represent a significant difference between EAC control group and other treated groups.
(#) represent a significant difference between DOX treated group and other treated groups.
While DOX elevated ALT and creatinine, CQ and GMF treated groups showed significant decreases in ALT, AST, urea and creatinine levels in comparison to those in DOX treated group. DOX plus CQ combination significantly decreased urea level when compared to EAC control group, while the ALT and creatinine were significantly increased. Effect of tested drugs on biochemical parameters was shown in table 5.
Table 5: Effect of DOX, CQ, GMF and their combinations on biochemical parameters.
| Treatment | ALT (U/L) | AST (U/L) | Urea
(mg/dl) |
Creatinine
(mg/dl) |
| Normal control | 14.6±1.2 | 41.6±0.88 | 39.1±1 | 0.42±0.01 |
| EAC control | 34±0.57 | 126.2±3.76 | 99.4±0.53 | 0.81±0.01 |
| Doxorubicin | 43±0.57** | 124.7±3.7 | 79.6±0.88** | 1±0.013** |
| Chloroquine | 21±0.57** # | 44.3±1.76** # | 46.4±0.8** # | 0.71±0.008# |
| Gemifloxacin | 18.3±0.66** # | 41.6±0.66** # | 62.3±1.4** # | 0.74±0.02# |
| DOX plus CQ | 65.3±2.9** # | 118.7±0.88 | 80±0.57** | 1.03±0.02** |
| DOX plus GMF | 36.3±1.76 | 109.9±3** # | 83.4±1.7** | 1.5±0.057** # |
Data are represented as Mean± SE (5animals/group).
(*) represent a significant difference between EAC control group and other treated groups.
(#) represent a significant difference between DOX treated group and other treated groups.
In the present study, DOX treated mice showed liver with marked inflammatory infiltrate, necrosis and hydropic degeneration. The kidney showed marked congestion and mild tubular degeneration. The heart showed marked edema, congestion and moderate degeneration of myocytes. The combinations induced less pathological lesions than DOX alone. Mice treated with DOX and CQ combination showed liver with minimal hydropic degeneration. The kidney showed minimal congestion, minimal tubular degeneration. Regarding to the heart, the myocytes were arranged into fascicles with striations and peripheral oval vesicular nuclei separated by minimal edema. Effects of these drugs on heart, liver and kidney histology were shown in Fig 1, 2 and 3.
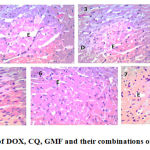 |
Figure 1: Histopathological effect of DOX, CQ, GMF and their combinations on the heart tissue, H&E stain, ×400. |
Normal control group (1), CQ treated group (2), GMF treated group (3), DOX treated group (4), CQ and DOX treated group (5), GMF and DOX treated group (6) and EAC control group (7).
(D) for degeneration of myocytes, (E) for edema and ( C) for
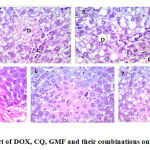 |
Figure 2: Histopathological effect of DOX, CQ, GMF and their combinations on the liver tissue, H&E stain, ×400. |
Normal control group (1), CQ treated group (2), GMF treated group (3), DOX treated group (4), CQ and DOX treated group (5), GMF and DOX treated group (6) and EAC control group (7).
(D) for hydropic degeneration and ( C) for congestion.
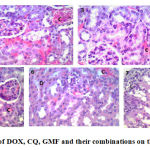 |
Figure 3: Histopathological effect of DOX, CQ, GMF and their combinations on the kidney tissue, H&E stain, ×400. |
Normal control group (1), CQ treated group (2), GMF treated group (3), DOX treated group (4), CQ and DOX treated group (5), GMF and DOX treated group (6) and EAC control group (7).
(D) for tubular degeneration,( L) for lymphocytic infiltration and ( C) for congestion.
Discussion
Doxorubicin is one of the most effective anticancer agents, used for different hematopoietic and solid cancers. DOX is one of the Topo II inhibitors that include anthracyclines, actinomycins and ellipticines (17). Quinolones are also Topo II inhibitors. Plus the antibacterial activities of quinolones, some members of this group effectively inhibit the eukaryotic Topo II enzymes (5, 6).
Cancer cell can protect itself against the presence of chemotherapy, nutrient deprivation and hypoxia through autophagy, that facilitate resistance to anticancer drugs (18). CQ sensitize resistant tumor cells to the toxic effects of anticancer agents by maintaining high concentrations of the chemotherapeutic agent intracellular (7).
The in-vitro study
Doxorubicin cytotoxicity on various cell lines, including the tested MCF7 and A549 cell lines, may be attributed to induction of Topo II- mediated DNA breaks and induction of apoptosis (19). The combination of DOX plus CQ was more effective than DOX alone. The inhibition of DOX efflux from the cell by CQ increased the cytotoxic effect of DOX on MCF7 and A549 cells. Similar findings was reported by Kvačkajová-Kišucká, Barančík [8] who studied the drug transporting system in relation to neoplasm-drug resistance.
In the same direction, DOX plus GMF was found to be more effective than DOX alone. Fluoroquinolones have anticancer properties mediated by the inhibition of DNA Topo II and DNA polymerase enzymes (20). Williamson et al. (21) reported that quinolone antibacterials inhibits metnase-dependent DNA repair, so they can increase the sensitivity of cancer cells and a xenograft tumors to chemotherapy.
DOX treatment caused generation of free radicals and oxidative damage to MCF7 cells. Although, both CQ and GMF induced some elevation in the level of free radicals in MCF7 cells, these elevations were not distinct as DOX did. These free radicals have been reported to contribute to the production of lipid peroxidation and damage of cell membrane leading to cell death (22). However both CQ and GMF showed some oxidative stress on MCF7 cells, the improvement of oxidative stress due to using of drug combination could be attributed to the using of half the dose of DOX. Such reduction of DOX dose lowered the oxidative stress and enhanced the cytotoxic effects of the combinations as shown in table 1.
The in-vivo study
Unlikely, DOX treatment decreased the MST when compared to EAC group. Similary, Ozols et al. (23) studied the penetration of DOX into tumor cells after i.v. injection in mice with a transplantable ovarian cancer, and found that DOX-specific intranuclear fluorescence was undetectable at any time following i.v. injection.
Mice treated with the combination of DOX and GMF showed significant increase in ILS% when compared to DOX treated mice. Infection is the second cause of death in breast cancer patients, followed by metastasis to various organs, thus quinolones may be an effective antibiotic in cancer patients (24). In the current study, CQ treated group significantly increased MST when compared to DOX treated group. Supra-additive cytotoxic activity induced by CQ combination could be explained by the blockage of autophagy (25).
The EAC control mice showed a significant increase in RBCs, Hb, HCT, and WBCs when compared to the normal control mice. These results may be attributed to the heamoconcentration caused by formation of intraperitoneal ascitis fluid. Ascitis induced-hemoconcentration resulted from the excessive fluid shifts away from blood vessels (26). DOX treated animals showed a significant decrease in RBCs, Hb, HCT and WBCs level when compared to EAC mice. Accumulation of DOX into nucleated blood cells would explain its acute hematological toxicity (22).
Mice treated with DOX showed a significant increase in ALT, AST, urea and creatinine levels when compared to normal control group. This may be attributed to the cell damage induced by DOX, as the metabolism of DOX occurs in all normal cells, especially in the liver, kidney and red blood cells (22). CQ and GMF showed significant decrease in ALT, AST, urea and creatinine levels when compared to DOX treated group. These reductions indicate less hepato-renal toxicities of GMF and CQ than DOX. Which in turn, elucidate the less toxicity of the combination therapy than DOX monotherapy.
Regarding the histopathological picture, the combinations were less toxic than DOX alone. Such improvement confirms our suggestion that the DOX combined therapy is less toxic than DOX alone. The outcomes of this study are; CQ and GMF enhance the antineoplastic effect of DOX and reduce its toxicities.
Conclusion
Cancer is among the leading causes of death worldwide. Currently, there is a growing interest in combining anticancer drugs to maximize their efficacy and minimize their side effects. We studied the synergistic effect of combining the anticancer drug, DOX, either with CQ or GMF on MCF7 and A549 cancer cell lines, and EAC bearing mice.
CQ and GMF enhanced the cytotoxic effect of DOX on MCF7 and A549 cells as the combinations showed a lower IC50 than DOX alone. The combinations decreased the oxidative damage caused by DOX as they significantly increased SOD and GSH and decreased MDA levels in MCF7 cells.
DOX was not effective anticancer drug in liquid EAC model when injected through i.v. route. The combination therapy significantly increased ILS% when compared to DOX treated group, also ALT and creatinine were significantly increased when compared to those measured in DOX treated group.
Further studies are required to understand molecular mechanism of anticancer effect of doxorubicin, chloroquine and gemifloxacin.
Acknowledgement
All authors appreciated the kind support of Pharmacology Department, Faculty of Veterinary Medicine, Suez Canal University.
Conflict of Interest
All authors ensure that there is no conflict of interest to disclose.
Funding source
This study received no fund from any funding organizations.
References
- Halazonetis, T.D., V.G. Gorgoulis, and J. Bartek. 2008.An oncogene-induced DNA damage model for cancer development. Science,319,1352-5.
CrossRef - Alapati, V., et al. 2012.In vivo anti-tumour activity of novel Quinazoline derivatives. European Review for Medical and Pharmacological Sciences,16,1753-64.
CrossRef - Taatjes, D.J., et al. 1998.A redox pathway leading to the alkylation of nucleic acids by doxorubicin and related anthracyclines: application to the design of antitumor drugs for resistant cancer. Current pharmaceutical design,4,203-218.
CrossRef - Drlica, K. and X. Zhao. 1997.DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiology and Molecular Biology Reviews,61,377-92.
CrossRef - Coughlin, S.A., et al. 1995.Mechanism of action and antitumor activity of (S)-10-(2,6-dimethyl-4-pyridinyl)-9-fluoro-3-methyl-7-oxo-2,3-dihydro-7 H- pyridol[1,2,3-de]-[1,4]benzothiazine-6-carboxylic acid (WIN 58161). Biochemical Pharmacology,50,111-22.
CrossRef - Clement, J.J., et al. 1995.Biological characterization of a novel antitumor quinolone. Cancer Research,55,830-5.
- Djordevic, B., C. Lange, and M. Rotman. 1992.Potentiation of radiation lethality in mouse melanoma cells by mild hyperthermia and chloroquine. Melanoma research,2,321-326.
CrossRef - Kvačkajová-Kišucká, J., M. Barančík, and A. Breier. 2001.Drug transporters and their role in multidrug resistance of neoplastic cells. General Physiology and Biophysics,20,215-237.
- Skehan, P., et al. 1990.New colorimetric cytotoxicity assay for anticancer-drug screening. Journal of the National Cancer Institute,82,1107-1112.
CrossRef - Mosmann, T. 1983.Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of immunological methods,65,55-63.
CrossRef - Salama, S.M., et al. 2013.Protective activity of Panduratin A against Thioacetamide-induced oxidative damage: demonstration with in vitro experiments using WRL-68 liver cell line. BMC complementary and alternative medicine,13,1.
CrossRef - Eralp, Y., et al. 2004.Doxorubicin and paclitaxel enhance the antitumor efficacy of vaccines directed against HER 2/neu in a murine mammary carcinoma model. Breast Cancer Research,6,1.
CrossRef - Rathos, M.J., et al. 2013.Potentiation of in vitro and in vivo antitumor efficacy of doxorubicin by cyclin-dependent kinase inhibitor P276-00 in human non-small cell lung cancer cells. BMC cancer,13,1.
CrossRef - Hiben, M.G., et al. 2016.Evaluation of Senna singueana leaf extract as an alternative or adjuvant therapy for malaria. Journal of traditional and complementary medicine,6,112-117.
CrossRef - Alkorta, M., et al. 2005.In vivo activity of gemifloxacin, moxifloxacin and levofloxacin against pneumococci with gyrA and parC point mutations in a sepsis mouse model measured with the all or nothing mortality end-point. International journal of antimicrobial agents,25,163-167.
CrossRef - Plowman, J., et al. 1997.Human tumor xenograft models in NCI drug development. 101-125.
CrossRef - Minotti, G., et al. 2004.Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacological reviews,56,185-229.
CrossRef - Kondo, Y., et al. 2005.The role of autophagy in cancer development and response to therapy. Nature Reviews Cancer,5,726-34.
CrossRef - Nitiss, J.L. 1998.Investigating the biological functions of DNA topoisomerases in eukaryotic cells. Biochimica et Biophysica Acta,1400,63-81.
CrossRef - Paul, M., et al. 2007.The anti-cancer effects of quinolone antibiotics? European Journal of Clinical Microbiology & Infectious Diseases,26,825-831.
CrossRef - Williamson, E.A., et al. 2012.Targeting the transposase domain of the DNA repair component Metnase to enhance chemotherapy. Cancer research,72,6200-6208.
CrossRef - Speth, P., Q. Van Hoesel, and C. Haanen. 1988.Clinical pharmacokinetics of doxorubicin. Clinical pharmacokinetics,15,15-31.
CrossRef - Ozols, R.F., et al. 1979.Pharmacokinetics of adriamycin and tissue penetration in murine ovarian cancer. Cancer research,39,3209-3214.
CrossRef - Cho, S.Y. and H.Y. Choi. 1980.Causes of death and metastatic patterns in patients with mammary cancer: ten-year autopsy study. American journal of clinical pathology,73,232-234.
CrossRef - Abdel-Aziz, A.K., et al. 2014.Chloroquine as a promising adjuvant chemotherapy together with sunitinib. Science Proceedings,1,
CrossRef - Khosropour, R., F. Lackner, and M. Zimpfer. 1983.Hemoconcentration caused by excessive intraoperative ascites production in ulcer bleeding. Anasth Intensivther Notfallmed,18,34-6.
CrossRef








