Khalida I. Noel1* , Mustafa M. Ibraheem1, Basim S. Ahmed2, Ahmed F. Hameed1, Nibras H. Khamees1 and Sameh S. Akkila1
, Mustafa M. Ibraheem1, Basim S. Ahmed2, Ahmed F. Hameed1, Nibras H. Khamees1 and Sameh S. Akkila1
1Department of Human Anatomy, College of Medicine, Mustansiriyah University, Baghdad, Iraq.
2Department of Pathology, College of Medicine, Mustansiriyah University, Baghdad, Iraq.
Corresponding Author’s Email: dr.khalidanoel@uomustansiriyah.edu.iq
DOI : https://dx.doi.org/10.13005/bpj/1769
Abstract
Benign and malignant prostatic diseases are generally well-known in the world. Accordingly, this research is planned to assess the immunohistochemical analysis of CD133 and CD166 in the prostatic epithelium in samples of benign prostatic hyperplasia (BPH) and normal looking epithelium around prostatic adenocarcinoma samples (PCa) and to explore the opportunity of malignant alterations in benign tissue. The prostate samples were divided into 2 groups; 50 BPH samples, and 50 normally looking tissue surrounding prostatic carcinoma samples (NPCA). The samples were treated for immunohistochemical examination of CD133 and CD166. Over expression of CD133 appeared in the BPH group which was statistically significant as compared to NPCA group. Conversely, over expression of CD166 stem cell marker in NPCA group than BPH group as it was significant statistically. CD166 is a stem cell marker for tissue tumorigenicity, while the positive expression of CD133 is not of value for cancer initiation.
Keywords
BPH; CD133; CD166; Immunohistochemistry; Prostatic Cancer
Download this article as:| Copy the following to cite this article: Noel K. I, Ibraheem M. M, Ahmed B. S, Hameed A. F, Khamees N. H, Akkila S. S. CD133 and CD166 Expression Predicting the Possibility of Prostatic Cancer Development in Cases of BPH. Biomed Pharmacol J 2019;12(3). |
| Copy the following to cite this URL: Noel K. I, Ibraheem M. M, Ahmed B. S, Hameed A. F, Khamees N. H, Akkila S. S. CD133 and CD166 Expression Predicting the Possibility of Prostatic Cancer Development in Cases of BPH. Biomed Pharmacol J 2019;12(3). Available from: https://bit.ly/2kKXP85 |
Introduction
Prostate is a male exocrine gland, and a portion of the male reproductive system.1 The adult human prostate tissue weighs roughly 20 g, and is 3 cm in length, 4 cm in width, and 2 cm in depth.2 The prostate expands with age of men; prostate enlargement is also related with appearance of benign prostatic hyperplasia (BPH).3 Prostate carcinoma is one of the very prevalent tumor in males of developed countries. There were 1.3 million newly diagnosed cases in 2018.4
BPH is a chronic illness described by prostatic enlargement, which manifested as lower urinary tract disorders.3 There are numerous factors which can effect for the development of BPH, such as metabolic syndromes, genetics and lifestyle. BPH is not supposed to be a straight risk factor or a pre-cancerous period in prostate cancer,5 but there are countless genetic, hormonal, and inflammatory causes have all been shared to be known pathophysiological dynamic mechanisms for the growth of both BPH and prostatic carcinoma (PCa), thus connecting these diseases together. Yet, on a cellular and molecular level, till now there is no revision shown that the change of BPH tissue has later transformed into an oncological ailment which is the core of the aims of this inquiry. Furthermore, the particular pathways of these prostatic diseases have yet to be fully assumed. This is crucial to enhance future management strategies for both diseases.6
Using stem cell markers like CD133 and CD166 can give a hint about the performance of the epithelial cells of prostatic gland in both BPH and normally looking tissue surrounding prostatic carcinoma, which specify that these cells have a stem cell-like comportment. The presence of CD133+ cells suggested that a high quantity of these tumor cells related to early lymph node metastasis, advanced cancer stages, and more poorly differentiated cancers.7,8 Whereas, the expression of CD166 by prostate stem cells propose the possibility of using this cell surface molecule in targeted therapies of human prostate tumors.9
Aims of the Study
This research directed to estimate the behavior of benign prostatic hyperplasia and the likelihood of alteration to cancerous lesion which requisite follow up later. In addition, to consider the role of the immunohistochemical (IHC) reaction types of glandular epithelium in normal tissue adjacent to prostatic carcinoma by using CD133 and CD166 markers as analyst to aid in choosing the best management for prostatic carcinoma. Finally, to compare the expression pattern of these markers in normal tissue adjacent to cancer and benign prostatic hyperplasia.
Methods
Patients
The present work was enrolled during the period extended from February 2018 to March 2019 in Al- Yarmouk teaching hospital (histopathology unit) and two private histopathological labs. The study was conducted on human prostatic tissue specimens received from patients attending the abovementioned hospital and labs.
A total number of 100 specimens were selected for the study, some were prospective, with a majority of retrospective samples obtained from archives of histopathology units of those labs and hospital.
The specimens were divided as follows:
Fifty primary prostatic carcinoma tissue samples were obtained from surgical resection of the prostate, biopsy was taken from normal tissue adjacent to the primary prostatic carcinoma (NPCA) (normal samples ≥ 5 cm distant from the cancer)10 and fifty tissue samples of benign prostatic hyperplasia (BPH) were obtained from transurethral resection surgery.
Patients were divided into two groups (table 1), according to case type, namely normal adjacent to cancer including 50 patients proved to have prostatic adenocarcinoma, their age ranged from 55-82 years with mean age 70 years. The second 50 patients had BPH and their age range between 60-86 years with mean age 71 years.
Ethical approval for the study was obtained from the ethical board of Al-Yarmouk teaching hospital. The pathological diagnosis of prostatic carcinoma was confirmed by reviewing a freshly prepared hematoxylin and eosin stained slides.
Table 1: Group categories by type and number
| Percentage | No. of patients | Mean age | Age (years) | Type | Group |
| 50% | 50 | 71 | 60 – 86 | BPH | I |
| 50% | 50 | 70 | 55 – 82 | NPCA | II |
| 100% | 100 | Total |
Immunohistochemistry
For each sample, 3 serial sections were taken, each with 4 micrometers thickness. The first serial section was placed on an ordinary slide and stained by hematoxylin/eosin stain to confirm the diagnosis and to determine the histological types and grades for the tumor. The second section was placed on positively charged slide for immunohistochemical staining with anti-CD133 antibody (Primary antibody from Abnova, Entrez GeneID 8842, Code PAB12663). The third section was treated with anti-CD166 antibody (Primary antibody from Abnova, Clone 10F1G12, Code MAB10485). Secondary antibody detection kit (Abcam, code ab64261, rabbit specific HRP/DAB) was used.
Formalin fixed samples and paraffin implanted tissue sections were dewaxed using xylene, and progressively hydrated. Antigen retrieval was done by pressure cooking using citrate buffer for 20 minutes. The primary anti-CD133 and anti-CD166 antibodies were diluted 1:200 using a background reducing dilution buffer (Abcam, code ab64211) and kept warm at room temperature for 30 minutes.
Detection performed by labeled streptavidin-biotin from Abcam secondary detection kit, followed by DAB and chromogen staining. The slides were quickly counterstained with hematoxylin, hydrated and mounted by DPX11.
Evaluation of the Immunohistochemical Staining
For CD133, all tissue samples of NPCA, and BPH were assessed without prior knowledge, and correlated with the age of the patient. It showed both cytoplasmic and membranous staining with more pronounced membranous and nuclear staining in some of the samples.
Evaluation of anti-CD166 antibody, all tissue samples of NPCA, and BPH were assessed blindly, and interrelated with the age of the patient. It showed both cytoplasmic and membranous staining with more pronounced membranous staining.12,13
Staining percentage and intensity for CD133 and CD166 were calculated as follows14,15:
Staining intensity was scored: 0 (no staining), 1+ (weak), 2+ (moderate) and 3+ (strong).
Extent of staining (percentage) was categorized by percentage: 0 = nil, 1= < 10 % of cell stained positively, 2= 10-50 %, 3= 51-80 %, 4= > 80 %
Statistical Analysis
Statistical analysis was performed by using the SPSS – (Statistical Packages for Social Sciences) V18. Categorical variables were evaluated by measuring the percentage, mean, and range (min-max values). The qualitative data were verified using Pearson Chi–square test (X2 –test), and independent sample t-test.
Results
Comparison of IHC marker expressions of CD133 and CD166 according to sample type
Regarding the intensity of markers staining and by using Pearson’s Chi-square test. In the BPH group, CD133 had the most intense response (3+, 44%) and none of the samples had a (0) response, while CD166 had the least response (1+, 48%) and none of the samples had a (3+) response (fig. 4, fig. 5, fig. 6, fig. 12, fig. 13, fig. 14). In the NPCA group, CD166 had the most intense response (3+, 48%) while the negative response was only 8%. On the other hand, 56% of cases in NPCA group obtained weak expression (1+) of CD133, but 0% of cases reflected the negative (0) expression of CD133 (fig. 8, fig. 9, fig. 10, fig. 15, fig. 16, fig. 17, fig. 18). Significant difference were obtained in between the two groups and markers as p-value= 0.000 (table 2 and figure 1).
For comparison of percentage of marker staining and by using t-test, the staining percentage was significantly different for the markers in each prostatic disorder. In the BPH group, CD133 was the mostly expressed marker in comparison to CD166 (p-value=0.019). In the NPCA group, CD166 was the mostly expressed marker in comparison to CD133 (p-value=0.027). CD133 was significantly more expressed in BPH group compared to NPCA group while CD166 had the opposite expression as shown on table 3 and figure 2.
The differences in total score of IHC marker expressions was assessed by t-test, and there was a statistical significance of different markers according to prostatic disorder. CD133 had the highest score in the BPH group in comparison to the NPCA group and the other marker (p-value=0.013). In the NPCA group, CD166 had the highest scores compared to the other marker in the same group and the same marker in the BPH group as p-value=0.031 (table 4 and figure 3).
CD133 was expressed clearly in the cell membrane and cytoplasm of prostatic cells in both groups, in addition a few cases appeared with nuclear association in BPH and NPCA groups as appeared in fig. 7 and fig. 11. While CD166 was appeared in cell membrane and cytoplasm of the cells in both groups.
Table 2: samples & marker type in relation to staining intensity (chi square)
| Staining Intensity | Sample Type | p-value | |||
| NPCA | BPH | ||||
| CD133 | CD166 | CD133 | CD166 | ||
| 0 | 0% | 8% | 0% | 46% | 0.000 |
| 1+ | 56% | 8% | 18% | 48% | |
| 2+ | 32% | 36% | 38% | 6% | |
| 3+ | 12% | 48% | 44% | 0% | |
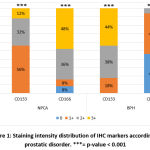 |
Figure 1: Staining intensity distribution of IHC markers according to prostatic disorder. |
Table 3: Sample & marker type in relation to staining percentage (t-test)
| Sample Type | IHC Marker | Staining percentage | P-value | |
| Mean | SD | |||
| BPH | CD133 | 78.7% | 18.9% | 0.019 |
| CD166 | 29.1% | 13.7% | ||
| NPCA | CD133 | 48.6% | 3.5% | 0.027 |
| CD166 | 83.6% | 7.5% | ||
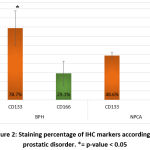 |
Figure 2: Staining percentage of IHC markers according to prostatic disorder. |
Table 4: Sample & marker type in relation to total score (t-test)
| Sample Type | IHC Marker | Total Score | p-value | |
| Mean | SD | |||
| BPH | CD133 | 1.86 | 0.8 | 0.013 |
| CD166 | 0.35 | 0.19 | ||
| NPCA | CD133 | 0.86 | 0.78 | 0.031 |
| CD166 | 2.08 | 0.9 | ||
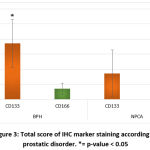 |
Figure 3: Total score of IHC marker staining according to prostatic disorder. |
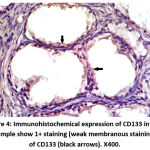 |
Figure 4: Immunohistochemical expression of CD133 in BPH sample show 1+ staining (weak membranous staining) of CD133 (black arrows). X400. |
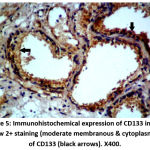 |
Figure 5: Immunohistochemical expression of CD133 in BPH sample show 2+ staining (moderate embranous & cytoplasmic staining) of CD133 (black arrows). X400. |
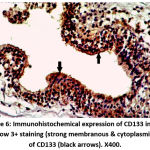 |
Figure 6: Immunohistochemical expression of CD133 in BPH sample show 3+ staining (strong membranous & cytoplasmic staining) of CD133 (black arrows). X400. |
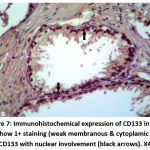 |
Figure 7: Immunohistochemical expression of CD133 in BPH sample show 1+ staining (weak membranous & cytoplamic staining) of CD133 with nuclear involvement (black arrows). X400 |
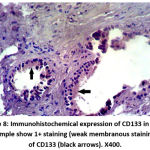 |
Figure 8: Immunohistochemical expression of CD133 in NPCA sample show 1+ staining (weak membranous staining) of CD133 (black arrows). X400. |
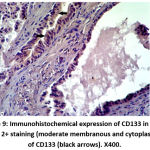 |
Figure 9: Immunohistochemical expression of CD133 in NPCA sample show 2+ staining (moderate membranous and cytoplasmic staining) of CD133 (black arrows). X400. |
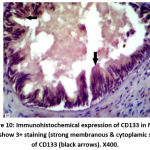 |
Figure 10: Immunohistochemical expression of CD133 in NPCA sample show 3+ staining (strong membranous & cytoplamic staining) of CD133 (black arrows). X400. |
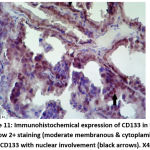 |
Figure 11: Immunohistochemical expression of CD133 in NPCA sample show 2+ staining (moderate membranous & cytoplamic staining) of CD133 with nuclear involvement (black arrows). X400 |
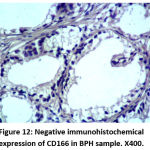 |
Figure 12: Negative immunohistochemical expression of CD166 in BPH sample. X400. |
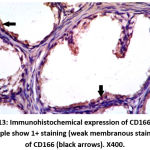 |
Figure 13: Immunohistochemical expression of CD166 in BPH sample show 1+ staining (weak membranous staining) of CD166 (black arrows). X400. |
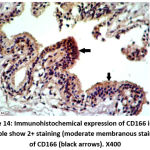 |
Figure 14: Immunohistochemical expression of CD166 in BPH sample show 2+ staining (moderate membranous staining) of CD166 (black arrows). X400 |
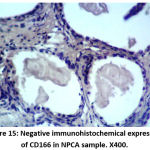 |
Figure 15: Negative immunohistochemical expression of CD166 in NPCA sample. X400. |
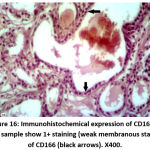 |
Figure 16: Immunohistochemical expression of CD166 in NPCA sample show 1+ staining (weak membranous staining) of CD166 (black arrows). X400. |
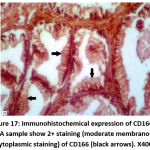 |
Figure 17: Immunohistochemical expression of CD166 in NPCA sample show 2+ staining (moderate membranous & cytoplasmic staining) of CD166 (black arrows). X400. |
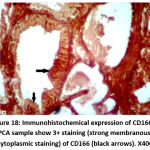 |
Figure 18: Immunohistochemical expression of CD166 in NPCA sample show 3+ staining (strong membranous & cytoplasmic staining) of CD166 (black arrows). X400 |
Discussion
Expression of CD133 in BPH and NPCA groups
As appeared in our results, CD133 stem cell marker had greater staining percentage in BPH than in NPCA group and was significant statistically. Also, CD133 expressed more intensely in BPH group than NPCA group and was also statistically significant. As a result, the total score of expression of CD133 showed statistically significant higher levels in BPH group than NPCA group. On other hand, 44% of samples in BPH group showed strong expression of CD133, whereas, 56% of NPCA group samples showed weak appearance of CD133 stem cell marker. These finding are in agreement with other researchers.16,17 All of these researchers showed the expression of CD133 positive cells in the prostatic epithelial cells in benign18 and malignant status of the epithelium19 as well as in the normal looking epithelial tissues.20
CD133 was found to be expressed in normal epithelial tissues of different organs such as the hematopoietic system,21 the prostate,22 the pancreas,23 and the kidney.24
Also CD133 is expressed in the benign epithelial conditions such as BPH18 and other benign conditions like benign tumors of skin with apocrine differentiation.19 In addition to the expression CD133 in malignant conditions like breast cancer,25 colorectal carcinoma,26 bladder carcinoma27 and prostatic cancer.18
Moreover, antibodies against CD133 have been designed for the separation and identification of a putative populace of tumor initiating cells or cancer stem cells (CSCs) in many of human carcinomas,28,29 and in malignant melanoma.30 In glioma, the amplified number of CD133 positive cancer cells, in addition to the existence of clusters of these cells, has been suggested as a main prognostic factor, autonomous of other features such as tumor grade.31 On the other hand, other readings, using altered and novel anti-CD133 clones, have proposed that the appearance of CD133 is not restricted to stem and progenitor cells32,33 and appears to be expressed in adult epithelial tissue cells of mouse and human.34,35
According to our results, all samples of both BPH and NPCA groups are CD133 positive and no negative expression were obtained in both groups. The explanations for these positivity is that: CD133 is a stem cell marker of normal, benign and malignant epithelial cells,12 but the intensity of CD133 expression was different in both groups. In BPH group; 44% of samples showed strong positivity, on the other hand, 56% of samples in NPCA group expressed CD133 weakly. The reason for this different positive intensity of expression is associated to differential attraction of the diverse antibodies to different glycosylated types of CD133. So the glycosylation may altered depending on the stage of cellular differentiation,19 or it can be changed during the path of malignant transformation.36 The other reason for over expression of CD133 in BPH samples is correlated to presence of inflammatory cell populations in BPH samples,17 which may be accompanying with the hyperplastic changes37 and so the intensity of staining is stronger than normal tissue around cancer in NPCA group which appeared low as explained by other researchers.17 In contrast, Miyazawa et al.,18 suggested that there were no clear reason for the different staining intensity between benign and cancer patients.
CD133 was lately established to undergo differential glycosylation in colon CSCs as paralleled with differentiated cancer cells.38 This occurrence would clarify some conflicting explanations that have been described when the CD133 protein and mRNA expression designs were linked.39 As newly suggested by Kemper et al., differential glycosylation of the extracellular domain of CD133 can armor the epitope from recognition by IHC using the antibody CD133.38
CD133 was considered as a marker of tumor initiating cells in many cancers,25 one of these cancers is the prostatic carcinoma. Based on these information, the tumor cells were allocated in to CD133+ and CD133- cells, that CD133+ cells showed stem like features,26 while CD133- cells did not. According to these data, the great number of CD133+ tumor cells is related with early lymph node metastasis, advanced cancer stages and poorly differentiated tumors40,41 and so exhibited resistance to management and poor survival26. Conversely, other researchers clarified that the tumorigenic potential did not exist in the CD133+ stem cells but was constantly detected in the CD133- populace.42 These facts established that benign basal cells contain cells of origin of prostate cancer and recommended that proliferative CD133- basal cells are more vulnerable to tumorigenesis if compared to CD133+ stem cells.43 Tumorigenic potential did not arise from positive CD133 stem cells; but might be appeared in the negative CD133 populace.44 Certainly, The resistance of CD133 positive cells to chemotherapy were more than CD133 negative cells in glioma and hepatocellular cancer, proposing CD133 as a probable marker of cancer stem cells.45,46 It has been displayed that prostate tumor derived CD133 positive cells are displaying self-renewal and widespread proliferation irrespective of the tumor grade.16 These conclusions confronted the existing acceptance that normal stem cells and cells of origin of cancer are the similar cell type(s).42 Thorough studies need to be performed to learn more about the role of CD133 in PCa origination.
Other researchers found that the significance of CD133 expression may be attenuated by using it with other stem cell markers to confirm the presence of stem cell like activity in prostatic epithelial cells.20
Expression of CD166 in BPH and NPCA groups
Our results showed that the percentage of CD166 expression in the NPCA group was higher than BPH group with statistical significance. Additionally, the strong intensity of CD166 staining was appeared in the NPCA group samples, were 48% of samples expressed 3+ level of intensity which was statistically significant as compared to BPH group with weak intensity (1+) for 48% of samples. As a result, statistically significant higher total score obtained in NPCA group as compared to BPH group. These results are agreed with the findings of other researchers.47,48
CD166 expression is commonly existing in most epithelial tissues and related carcinomas.49 It is important in tumor development and invasion.50 The molecular activity of CD166 is controlled through shedding of its extracellular domain.51
CD166 has been mentioned in few revisions as a significant potential biomarker for prostatic carcinoma,9 although it is functionally and clinically connected to many other cancers in the body.48,52
According to Kristiansen et al.,47 CD166 was usually expressed in normal prostatic epithelia which showed a mainly membranous and weak cytoplasmic staining of secretory cells with no staining of basal cells and stromal appearance was not detected, the staining was commonly homogenous which conclude the cell adhesive criteria of CD166 molecule. It involves both the stem cells and progenitor populations which is a likely function for CD166 to preserve the reliability of the stem cell niche by preserving the epithelial microenvironment.49 CD166 is commonly elaborate in morphogenesis of tubular structures, regardless of endothelial or epithelial source. This mechanism would clarify the low CD166 appearance in normal prostatic glands, which illustrate a very low level of proliferation.47 CD166 also appeared in the hyperplastic glands with weak intensity.47
CD166 is a tumor initiating and cancer stem cell markers48 and it is up regulated in prostatic carcinoma,53 give the reason that when addition of CD166; augmented sphere forming activity in prostatic tumor cell lines and in human prostatic cancer specially castration resistant prostatic cancer (CRPC) samples.43 Consequently, CD166 may enhance both human prostate tissue stem/progenitor cells and (CRPC) cells.9 Prostate stem/progenitor cells function in glandular development and preservation; they may be marks for tumor initiation, so classification of these cells may be of therapeutic value.54 Cells from detached tissues that form spheres in vitro often characterize stem/progenitor cells. A subclass of human prostate cells that custom spheres are accomplished for self-renewal and tissue regeneration.55
Normal human prostate comprises three dissimilar sorts of cells, that is luminal secretory, basal and neuroendocrine cells. Subsequently, human prostate cancer is described by loss of basal cells and growth of luminal cells, numerous animal models suggest that luminal specific progenitors are the causes of initiating prostate cancer.56 Though, using the tissue regeneration methodology, basal cells have verified to be very effective oncogenic targets for human prostate cancer initiation.57,58 Remarkably, Choi et al., confirmed that adult murine prostate basal and luminal cells are self-sustained lineages that both of them can assist as oncogenic targets for prostate cancer initiation.59
In our results, this up regulation ad strong expression of CD166 in the normal tissue around prostatic cancer was obtained and this can be explained on the finding of Jones et al., that considered this normal tissue as a field cancerization zone because of the molecular alterations of the cells in the normal tissue adjacent to cancer and so express CD166 strongly and give the idea of presence of tumor initiating cancer stem-like cell in this tissue or even they are a cancer stem cells.60 The explanations for this field effect was described by many researchers as that tumor tissue and adjacent normal tissue,61 both of them exhibited significant up regulation of proliferation related genes including transcription factors,62 signal transducers and growth regulators and proposed that normal appearing prostate tissue can experience genetic modifications in response to or in expectation of morphologic cancer.63,64 This is an important prognostic feature which determine the suggestion of increased CD166 appearance with human prostate cancer metastasis and CRPC growth. Furthermore, CD166-high expressing subpopulation involves prostate stem/progenitor and cancer initiating cells.9
However, Kristiansen et al., 2003 showed that CD166 is over expressed in low grade tumor but is down regulated in high grade tumor, This difference might be clarified by altered biological characters of CD166 in cell adhesion of different cancers,65 another explanation for this difference of CD166 expression was that glandular (low-grade) carcinomas precise CD166 at higher levels, perhaps replicating ongoing growth and tubules development, however high-grade cancers increasingly wildness tubules growth in favor of cribriform, solid, or single-cell invasion designs.47 Other reasons included CD166 mRNA up regulation in low grade prostate cancer and progressive loss in high-grade lesions may be of important implication.66
So CD166 have a very important prognostic effects on prostatic carcinoma treatment and follow up. CD166 has also been recommended to show a serious part in numerous human carcinomas and play as a potential therapeutic target for cancer initiating cells,67 and may be for a proper surface marker for upcoming targeted drug delivery.68 Also can be applied to examine the efficiency of CD166 – mediated drug delivery to prostate cancer initiating cells in vivo, particularly during CRPC expansion.9
The weak expression of CD166 in 48% of samples of BPH may be of significant importance in determining whether there were a tumor initiating cancer stem-like cell or cancer stem cell in the hyperplastic tissue, Jiao et al.,9 supposed that CD166 was focally appeared in the benign adult prostate, CD166 might augment sphere-forming capability of benign primary human prostate cells in vitro and encourage the formation of tubule-like organizations in vivo. But, Weichert et al.,14 hypothesized that over expression of CD166 is a premature occurrence in malignant cell alteration in colon carcinogenesis, as it was established in all adenomas of the colon, which was reflected to be precursor lesions. According to these findings, further information and more molecular investigations may be needed to confirm these findings. In addition, in murine models, CD166 was up regulated in prostates after castration.9 These records specified that the amounts of stem cells in primary tumors or the patient circulation can be applied to recognize patients likely to experience a relapse and for whom very aggressive management is required.
IHC Expression of CD133 and CD166 in BPH and NPCA groups
CD133 is a cell membrane marker,44 but also expressed in the cytoplasm of the cells.69 Our results showed both cell membrane and cytoplasmic appearance of CD133 in both BPH and CA group of samples and this is agreed with the findings of Huwait et al.,44 In addition to membranous and cytoplasmic expressions of CD133, nuclear involvement also appeared in the nuclei of prostatic cells in BPH and normal tissue around cancer in the NPCA group. These results are similar to the findings of many researchers in breast cancer,12 lung cancer,69 hepatocellular carcinoma70 and colorectal cancer71 respectively. The explanation of this nuclear expression is controversial. Cantile et al., and Huang et al.,12,69 hypothesized that nuclear localization of CD133 may be a sign of poor prognosis in breast cancer and lung cancer, as they recognized that surface molecules, when travelling into the nucleus, can act as transcriptional regulators by interfering with molecular paths directly linked to the proliferation and differentiation of cancer cells. In contrast, Chen et al. and Lee et al., 70,71 hypothesized that cytoplasmic CD133 appearance was associated with poor prognosis, while nuclear CD133 appearance was considerably associated with positive prognosis. No previous study explained the nuclear expression of CD133 in BPH, but we must take this nuclear expression of CD133 in some of BPH cases on considerations that it might give us a hint for any transformation and cancer development risk, since some researchers demonstrated a telomerase activity in BPH might change and therefore would obtain similar characters like those of normal looking cells around tumor and cancer cells and so the possibility of developing cancer is present and follow up is needed.72 So, further studies are needed for examining CD133 expression role in the development and progress of prostate carcinoma and its appropriateness as a prognostic biomarker.73
CD166 is a membranous marker also and cytoplasmic expression is appeared in our results and agreed with others.14,50 But some researchers hypothesized that membranous expression of CD166 is suggestive of poor survival of colorectal cancer.14 So, further prognostic and therapeutic stratification may be achieved according to CD166 localization.
Conclusions
This study provided suggestion that:
Over expression of CD166 in normal tissue around prostatic carcinoma than benign tissue in BPH.
Over expression of CD133 in benign tissue of BPH cases than normal tissue around prostatic carcinoma.
Increasing age is one of important common reasons of both BPH and prostatic carcinoma in addition to other epidemiological causes.
Possibility of changing in histology of benign tissue to malignant is still query and need more advanced investigation on molecular level, but follow up of suggestive BPH cases must take into consideration.
CD166 is a stem cell marker for tumor tumorigenicity, while the positive expression of CD133 is not of value for cancer initiation.
Acknowledgements
This work was not supported by research grants.
References
- Snell R. Clinical anatomy by regions 9th edition, Lippincott Williams & Wilkins, 2012: 275-277.
- Standring S. Gray’s anatomy. The anatomical basis of clinical practice 41th edition, Elsevier, 2016: 1266-1272.
- Yoo, T.K., Cho, H.J. Benign prostatic hyperplasia: from bench to clinic. Korean J Urol, 2012; 53, 139-148.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA cancer J Cli, in press., 2018.
- Luo, J., Duggan, D.J., Chen, Y., Sauvageot, J., Ewing, C.M., Bittner, M.L., Trent, J.M., Isaacs, W.B. Human prostate cancer and benign prostatic hyperplasia: molecular dissection by gene expression profiling. Cancer Res, 2001; 61, 4683-4688.
- Miah S, Catto J. BPH and prostate cancer risk. Indian J Urol., 2014; 30(2): 214–218.
- Mei W, Lin X, Kapoor A, Gu Y, Zhao K, Tang D. The Contributions of Prostate Cancer Stem Cells in Prostate Cancer Initiation and Metastasis. Cancer, 2019; 11(4), 434.
- Pop MG. Stem Cell Markers in Colon Cancer. Basic Principles and Practice in General Surgery, 2019; DOI: http://dx.doi.org/10.5772/intechopen.84315.
- Jiao, J., Hindoyan, A., Wang, S., Tran, L.M., Goldstein, A.S., Lawson, D., Chen, D., Li, Y., Guo, C., Zhang, B., Fazli, L., Gleave, M., Witte, O.N., Garraway, I.P., Wu, H. Identification of CD166 as a surface marker for enriching prostate stem/progenitor and cancer initiating cells. PLoS One, 2012; 7, e42564.
- Zhou F, Donmgu Y, Jjunlang, Xinliu Z, Chen HS, Zhang. Expression and prognostic value of tumor stem cell markers ALDH1 and CD133 in colorectal carcinoma. Int J Clin Exp Pathol., 2014; 7(6): 2976–2986.
- Mutlak SS, Hasan NA, AL-Hijazi AY. Biochemical And Immunohistochemical Evaluation Of Transforming Growth Factor- Beta1 And Tumor Necrosis Factor- Alpha In Dental Diseases. IJRPC, 2015; 5(4), 736-752.
- Irina Ch, Barbalan A, Pirici D, Margaritescu C, Saftoiu A. Stem Cells, Colorectal Cancer and Cancer Stem Cell Markers Correlation. Current Health Science Journal, 2014; Vol 40, No 3.
- Weichert W, Knosel T, Bellach J, et al. ALCAM/CD166 is overexpressed in colorectal carcinoma and correlates with shortened patient survival. J Clin Pathol, 2004;57:1160–4.
- Huang M, Zhu H, Feng J, Ni S, Huang J. High CD133 Expression in the Nucleus and Cytoplasm Predicts Poor Prognosis in Non-Small Cell Lung Cancer. Hindawi Publishing Corporation Disease Markers; 2015: 1-8. Article ID 986095, http://dx.doi.org/10.1155/2015/986095
- Burkhardt M, Mayordomo E, Winzer KJ, Fritzsche F, Gansukh T, Pahl S, Weichert W, Denkert C, Guski H, Dietel M, Kristiansen G. Cytoplasmic overexpression of ALCAM is prognostic of disease progression in breast cancer. Clin Pathol, 2006;59:403–409.
- Ugolkov AV, Eisengart LJ, Luan C, Yang XJ. Expression analysis of putative stem cell markers in human benign and malignant prostate. Prostate, 2011; 1;71(1):18-25. doi: 10.1002/pros.21217
- Zhang K, Zhou S, Wang L, Wang J, Zou Q, Zhao W, Fu Q, and Fang X. Current Stem Cell Biomarkers and Their Functional Mechanisms in Prostate Cancer. Int. J. Mol. Sci. 2016, 17, 1163; doi:10.3390/ijms17071163.
- Miyazawa K, Tanaka T, Nakai D, Morita N, Suzuki K. Immunohistochemical expression of four different stem cell markers in prostate cancer: High expression of NANOG in conjunction with hypoxia‑inducible factor‑1α expression is involved in prostate epithelial malignancy. Oncology Letters, 2014; 8: 985-992.
- Nam-Cha SH, Serrano-Vargas IR, Escario E, Azaña JM, Calero-Oliver R,Martín AG and Poblet E. CD133 Expression in Normal Skin and in Epithelial Cutaneous Tumors. Hindawi Publishing Corporation BioMed Research International, 2013; Volume 2013, Article ID 385604, 8 pages http://dx.doi.org/10.1155/2013/385604
- Trerotola M, Rathore S, Goel HL, Li J, Alberti S, Piantelli M, Adams D, Jiang Z and Languino LR. CD133, Trop-2 and α2β1 integrin surface receptors as markers of putative human prostate cancer stem cells. Am J Transl Res, 2010;2(2):135-144.
- Bauer N, Fonseca AV, Florek M et al. New insights into the cell biology of hematopoietic progenitors by studying prominin-1 (CD133). Cells Tissues Organs, 2008; 188, no. 1-2: 127–138.
- Richardson, G.D., Robson, C.N., Lang, S.H., Neal, D.E., Maitland, N.J., Collins, A.T. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci, 2004; 117, 3539-3545.
- Immervoll H, Hoem D, Sakariassen P, Steffensen OJ, and Molven A. Expression of the “stem cell marker” CD133 in pancreas and pancreatic ductal adenocarcinomas. BMC Cancer, 2008; vol. 8, article 48.
- Sagrinati C, Netti GS, Mazzinghi B et al. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. Journal of theAmerican Society of Nephrology; 2006; 17( 9): 2443–2456.
- Nadal R, Ortega3 FG, Salido M, Lorente JA, Rodıguez-Rivera M, Delgado-Rodrıguez M, Macia M, Ferandez A, Corominas JM, Garcıa-Puche JL, Sanchez-Rovira P, Sole F, Serrano MJ. CD133 expression in circulating tumor cells from breast cancer patients: Potential role in resistance to chemotherapy. Int. J. Cancer, 2013; : 133:2398–2407.
- Zhao Y, Peng J, Zhang E, Jiang N, Li J, Zhang Q, Zhang X, Niu Y. CD133 expression may be useful as a prognostic indicator in colorectal cancer, a tool for optimizing therapy and supportive evidence for the cancer stem cell hypothesis: a meta-analysis. Oncotarget, 2016; 7 (9): 10023- 10036.
- Sedaghat S, Gheytanchi E, Asgari M, Roudi R, Keymoosi H, Madjd Z. Expression of Cancer Stem Cell Markers OCT4 and CD133 in Transitional Cell Carcinomas. Appl Immunohistochem Mol Morphol, 2016; Volume 00, Number 00, www.appliedimmunohist.com
- Florek M, Haase M, Marzesco AM et al. Prominin- 1/CD133, a neural and hematopoietic stem cell marker, is expressed in adult human differentiated cells and certain types of kidney cancer. Cell and Tissue Research, 2005; 319(1):15–26.
- Okudela K, Woo T, Mitsui H, Tajiri M, Masuda M, Ohashi K. Expression of the potential cancer stem cell markers, CD133, CD44, ALDH1, and 𝛽-catenin, in primary lung adenocarcinoma—their prognostic significance. Pathology International, 2012; 62(12): 792–801.
- Klein WM, Wu BP, Zhao S, Wu H, Klein-Szanto AJP, Tahan SR. Increased expression of stem cell markers in Malignant melanoma. Modern Pathology, 2007; 20(1): 102–107.
- Zeppernick F, Ahmadi R, Campos B et al. Stem cell marker CD133 affects clinical outcome in glioma patients. Clinical Cancer Research, 2008; 14(1): 123–129.
- Weigmann A, Corbeil D, Hellwig A, Huttner WB. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proceedings of the National Academy of Sciences of the United States of America, 1997; 94(23): 12425–12430.
- Maw M A, Corbeil D, Koch J et al. A frameshift mutation in prominin (mouse)-like 1 causes human retinal degeneration. Human Molecular Genetics, 2000; 9(1): 27–34.
- Pfenninger CV, Roschupkina T, Hertwig F et al. CD133 is not present on neurogenic astrocytes in the adult subventricular zone, but on embryonic neural stem cells, ependymal cells, and glioblastoma cells. Cancer Research, 2007; 67(12): 5727–5736.
- Karbanová, J., Missol-Kolka, E., Fonseca, A. V., Lorra, C., Janich, P., Hollerová, H., et al. The stem cell marker CD133 (Prominin-1) is expressed in various human glandular epithelia. J. Histochem. Cytochem., 2008; 56, 977–993.
- Corbeil D, R¨oper K, Hellwig A et al., The human AC133 hematopoietic stem cell antigen is also expressed in epithelial cells and targeted to plasma membrane protrusions. Journal of Biological Chemistry, 2008; 275(8): 5512–5520.
- Chan SW. Pathology and Medical Therapy of Benign Prostatic Hyperplasia. The Hong Kong medical diary, 2011; 16(6): 4-8.
- Kemper K, Sprick MR, de Bree M, Scopelliti A, Vermeulen L, Hoek M, Zeilstra J, Pals ST, Mehmet H, Stassi G and Medema JP. The AC133 epitope, but not the CD133 protein, is lost upon cancer stem cell differentiation. Cancer Res., 2010; 70: 719-729.
- Bidlingmaier S, Zhu X, Liu B. The utility and limitations of glycosylated human CD133 epitopes in defining cancer stem cells. J Mol Med., 2008; 86:1025–1032.
- O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature, 2007; 445:106–110.
- Grosse-Gehling P, Fargeas CA, Dittfeld C, Garbe Y, Alison MR, Corbeil D, Kunz-Schughart LA. CD133 as a biomarker for putative cancer stem cells in solid tumours: limitations, problems and challenges. J Pathol., 2013; 229:355–378.
- Taylor, R.A. Toivanen, R. Frydenberg, M. Pedersen, J. Harewood, L. Australian Prostate Cancer Bioresource; Collins, A.T.; Maitland, N.J.; Risbridger, G.P. Human epithelial basal cells are cells of origin of prostate cancer, independent of CD133 status. Stem Cells, 2012; 30, 1087–109.
- Moltzahn F, Thalmann GN. Cancer stem cells in prostate cancer. Transl Androl Urol, 2013; 2(3):242-253.
- Huwait HF, Nassir AM, Abd Elmoneim HM, Kamel HFM, Toni ND, Babtain NA, Barhamain AS, Malibari AS, Munassar SF, Rawa RS and Kufiah AZ. Clinical Significance and Potential Utility of Cancer Stem Cell Markers: ALDH1A1 and CD133 in Prostate Tumors. International Journal of Cancer Research, 2018; 14 (1): 39-5.
- Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS. Analysis of gene expression and chemoresistance of CD133þ cancer stem cells in glioblastoma. Mol Cancer, 2006;5:67.
- Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133þ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene, 2008; 27(12):1749–1758.
- Kristiansen G, Pilarsky C, Wissmann C, Stephan C, Weissbach L, Loy V, Loening S, Dietel M, Rosenthal A. ALCAM/CD166 Is Up-Regulatedin Low-Grade Prostate Cancer and Progressively Lost in High-Grade Lesions. The Prostate, 2003; 54:34-43.
- Rajasekhar, V.K., Studer, L., Gerald, W., Socci, N.D., Scher, H.I. Tumour-initiating stem-like cells in human prostate cancer exhibit increased NF-kappaB signalling. Nat Commun, 2011; 2, 162.
- Levis TG, Powell AE, Davies PS, Silk AD, Dismule AD, Anderson EC, Swain JR, Wong MH. Charaterization of the intestinal cancer stem cell markers.CD166/ALCAM in the human and mouse gastrointestinal tract. Gastroenterology, 2011; 139(6) 2072-2082.
- Abdullah NM. Expression of Stem cell Markers CD44, ALDH1A1 and CD166 in Normal Tissue Adjacent to Colorectal Carcinoma in Sample of Iraqi Patients. Thesis. Mustansiriyah medical college., 2017.
- Lugli A, Iezzi G, Hostettler I, Muraro MG, Mele V, Tornillo L, Carafa V, Spagnoli G, Terracciano L, Zlobec I. Prognostic impact of the expression of putative cancer stem cell markers CD133, CD166, CD44s, EpCAM, and ALDH1 in colorectal cancer. Br J Cancer, 2010; 27;103(3):382-390.
- Tachezy M, Zander H, Gebauer F, Marx A, Kaifi JT, Izbicki JR, et al. Activated leukocyte cell adhesion molecule (CD166)-Its prognostic power for colorectal cancer patients. J Surg Res., 2012; 177(1):e15-20. doi: 10.1016/j.jss.2012.02.013.
- Stamey TA, Warrington JA, Caldwell MC, et al. Molecular genetic profiling of Gleason grade 4/5 prostate cancers compared to benign prostatic hyperplasia. J Urol, 2001;166:2171–7.
- Kwon OJ, Xin L. Prostate epithelial stem and progenitor cells. Am J Clin Exp Urol, 2014;2(3):209-218.
- Garraway, I. P., Sun, W., Tran, C. P., Perner, S., Zhang, B., Goldstein, A. S., et al. Human prostate sphere-forming cells represent a subset of basal epithelial cells capable of glandular regeneration in vivo. Prostate, 2010; 70, 491–501. doi: 10.1002/pros.21083.
- Wang, X., Kruithof-de Julio, M., Economides, K.D., Walker, D., Yu, H., Halili, M.V., Hu, Y.P., Price, S.M., Abate-Shen, C., Shen, M.M. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature, 2009; 461, 495-500.
- Lawson, D.A., Zong, Y., Memarzadeh, S., Xin, L., Huang, J., Witte, O.N. Basal epithelial stem cells are efficient targets for prostate cancer initiation. Proc Natl Acad Sci U S A, 2010; 107, 2610-2615.
- Goldstein, A.S., Huang, J., Guo, C., Garraway, I.P., Witte, O.N. Identification of a cell of origin for human prostate cancer. Science, 2010; 329, 568-571.
- Choi, N., Zhang, B., Zhang, L., Ittmann, M., Xin, L. Adult murine prostate basal and luminal cells are self-sustained lineages that can both serve as targets for prostate cancer initiation. Cancer Cell, 2012; 21, 253-265.
- Jones AC, Trujillo KA, Phillips GK, Fleet TM, Murton JK, Severns V, Shah SK, Davis MS, Smith AY, Griffith JK, Fischer EG, Bisoffi M. Early Growth Response1and Fatty Acid Synthase Expression is Altered in Tumor Adjacent Prostate Tissue and Indicates Field Cancerization. Prostate, 2012; 1; 72(11): 1159–1170.
- Chandran UR, Dhir R, Ma C, Michalopoulos G, Becich M, Gilbertson J. Differences in gene expression in prostate cancer, normal appearing prostate tissue adjacent to cancer and prostate tissue from cancer free organ donors. BMC Cancer, 2005; 5:45.
- Galluzzi CM, Maddala T, Falzarano SM, Cherbavaz DB, Zhang N, Knezevic D, Febbo PG, Lee M, Lawrence HJ, Klein EA. Gene expression in normal-appearing tissue adjacent to prostate cancers are predictive of clinical outcome: evidence for a biologically meaningful field effect. Oncotarget, 2016; 7(23):33855-33865.
- Trevino V, Tadesse MG, Vannucci M, Al-Shahrour F, Antczak P, et al. Analysis of Normal-Tumour Tissue Interaction in Tumours: Prediction of Prostate Cancer Features from the Molecular Profile of Adjacent Normal Cells. PLoS ONE, 2011; 6(3): e16492. doi:10.1371/journal.pone.0016492.
- Haaland CM, Heaphy CM, Butler KS, Fischer EG, Griffith JK, Bisoffi M. Differential gene expression in tumor adjacent histologically normal prostatic tissue indicates field cancerization. International Journal Of Oncology, 2009; 35: 537-546.
- Ohneda O, Ohneda K, Arai F, et al. ALCAM (CD166): its role in hematopoietic &endothelial development. Blood, 2001; 98:2134–42.
- Weidle UH, Eggle D, Klostermann S, Swart GWM. ALCAM/CD166: Cancer-related Issues. Cancer Genomics & Proteomics, 2010;7: 231-244.
- Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med, 2011; 17: 211–215.
- Roth A, Drummond DC, Conrad F, Hayes ME, Kirpotin DB, et al. Anti-CD166 single chain antibody-mediated intracellular delivery of liposomal drugs to prostate cancer cells. Mol Cancer Ther, 2007; 6: 2737–2746.
- Cantile M, Collina F, D’Aiuto M, Rinaldo M, Pirozzi G, Borsellino C, Franco R, Botti G, Di Bonito M. Nuclear localization of cancer stem cell marker CD133 in triple-negative breast cancer: a case report. Tumori., 2013; 99(5): e245-50.
- Chen YL, Lin PY, Ming YZ, Huang WC, Chen RF, Chen PM, Chu PY. The effects of the location of cancer stem cell marker CD133 on the prognosis of hepatocellular carcinoma patients. BMC Cancer, 2017;17:474.
- Lee YM, Yeo MK, Seong IO, Kim KH. Nuclear Expression of CD133 Is Associated with Good Prognosis in Patients with Colorectal Adenocarcinoma. Anticancer Res., 2018;38(8):4819-4826.
- Scates Dk, Muir GH, Venitt S, Carmichael PL. Detection of telomerase activity in human prostate: a diagnostic marker for prostatic cancer? British Journal of Urology, 1997; 80, 263–268.
- Adu-Addai B, Salam AB, Amin R, Wang H, Lin H, Ghebremedhin A, White J, Jaynes JM, Yates C. CD133 as a disparity stem cell biomarker in African Americans with late-stage prostate cancer. AACR Annual Meeting 2019; March 29-April 3, 2019; Atlanta, GA; 79(13).







