Vamsi Lavu1, V. Vettriselvi 2, V. Priyanka3, R. Suresh3 and S. K. Balaji3
1Periodontology, Faculty of Dental Sciences, Sri Ramachandra Institute of Higher Education and Research (DU).
2Human Genetics Faculty of Biomedical Sciences, Sri Ramachandra Institute of Higher Education and Research (DU).
3Department of Periodontology, Faculty of Dental Sciences, Sri Ramachandra Institute of Higher Education and Research (DU).
Corresponding Author E-mail: lavu.vamsi@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1684
Abstract
Periodontitis is a multi-factorial disease with bacterial origin. The progression is influenced by systemic disease, smoking, genetic factors. In the recent past epigenetic influences have been indentified on the candidate genes which code for proteins that play a role in the pathogenesis of the periodontal disease. Epigenetic mechanisms involve DNA methylation and histone modification. Till date, there is no existing data associating methylation status of the promoter region (-239, -245) of the TNF alpha gene and chronic periodontitis. The objective of this study was to compare the methylation status of CpG islands in the promoter region of TNF alpha (-239, -245) gene in the peripheral blood among subjects with healthy gingiva and chronic periodontitis. A case control study design involving 50 subjects (25 healthy and 25 subjects with chronic periodontitis) was performed. DNA was isolated from peripheral blood of all the subjects and bisulfate modification with methylation specific polymerase chain reaction (MS-PCR) was performed. The methylation status of the selected region of the Tumor necrosis factor alpha gene for both the test and control groups was evaluated and assessed. The mean Ct value for the methylation of control group was 24.36 and in the periodontitis group the mean Ct value was found to be 28.09. The higher the Ct value, the lower is the amount of methylation observed. The periodontitis group had a higher mean Ct value as compared to the control group; indicating a lower amount of methylation in the promoter region of the periodontitis group as compared to the control group. A lower level of TNF alpha gene promoter (-239,-245) methylation was observed in the periodontitis group as compared to the controls and this observation appears to support a de-methylation of the TNF alpha promoter in the periodontitis subjects. Further studies evaluating the transcript levels of TNF alpha needs to be performed to confirm the observations of this study.
Keywords
Epigenetics; Methylation; Periodontitis; Tumor Necrosis Factor Alpha Gene
Download this article as:| Copy the following to cite this article: Lavu V, Vettriselvi V, Priyanka V, Suresh R, Balaji S. K. Methylation Status of Promoter Region of Tumor Necrosis Factor Alpha Gene in Subjects with Healthy Gingiva and Chronic Periodontitis - A Pilot Study. Biomed Pharmacol J 2019;12(2). |
| Copy the following to cite this URL: Lavu V, Vettriselvi V, Priyanka V, Suresh R, Balaji S. K. Methylation Status of Promoter Region of Tumor Necrosis Factor Alpha Gene in Subjects with Healthy Gingiva and Chronic Periodontitis - A Pilot Study. Biomed Pharmacol J 2019;12(2). Available from: https://bit.ly/2YdWXHn |
Introduction
Chronic Periodontitis is primarily an inflammatory disease affecting the supporting tissues of the teeth and has an infectious origin.1 The disease initially starts off as an inflammation of the gums (gingivitis) and eventually progresses to involve the underlying bone (periodontitis).2 The disease progression however is not the same in all individuals. Susceptibility to development of periodontitis has been examined and several risk factors identified. The risk factors include systemic diseases such as diabetes mellitus, smoking, genetic factors. Amongst the genetic factors, several candidate gene polymorphisms have been examined for their association with periodontitis susceptibility.3,4 Chronic periodontitis being an inflammatory disease, several inflammatory mediators have been implicated in the initiation and progression of disease. TNF alpha represents one such key mediator which modulates the immune system and is also involved in connective tissue and bone destruction which takes place during chronic periodontitis.5
Epigenetics is defined as all the meiotically and mitotically inherited changes in the gene expression that are not encoded in the DNA sequence itself.6 Epigenetic modifications of chromatin and DNA have been recognized as important permissive and suppressive factors in controlling the expressed genome via gene transcription.7 Epigenetic changes play a major role in development by mechanism called as allelic silencing. Specific allelic form of a gene is suppressed resulting in shaping the development in a particular direction; the classical example being: X chromosome inactivation; wherein one of the two copies of the X chromosome in the females is silenced by epigenetic mechanisms.8 Of late, the role of the epigemone in controlling gene expression has been studied extensively in conditions such as cancer9 and inflammatory conditions.10 The environment also has been proposed to influence various systemic conditions through modification of the epigemone, a classical example being the influence of nutrition on systemic conditions.11 The role of modifications in the epigenome of genes controlling production of inflammatory cytokines has been studied and reported in rheumatoid arthritis wherein the methylation status of promoter region of the gene controlling TNF alpha production, has been reported to increase the TNF alpha levels contributing to severity of the rheumatoid arthritis.12
Epigenetic Changes in the Human Genome Can Involve Different Mechanisms Such As
a). Methylation of DNA sequences- mediated by methyl transferases, b). Acetylation of histone proteins – mediated by acetylases and de-acetylases. c) Methylation of histone proteins- mediated by histone methyl transferases. Studies have been done on the alteration of the methylation status of the promoter region of Interleukin 8 gene and gene regulating Cox-2 expression in chronic periodontitis as compared to healthy controls. Oliveira NF et al 2009.13 reported a higher frequency of hypomethylation in the promoter region of the Il-8 gene in subjects with chronic periodontitis as compared to healthy controls and the hypomethylation was associated with elevated IL-8 m-RNA levels in oral cells. Zhang S et al 2010,14 reported a hypermethylation of the promoter region of the gene PTGS2, which also co-related with a down regulation of Cox-2 levels in chronic periodontitis subjects. Zhang S et al 2010,15 observed a hypo-methylation of the CpG islands in the promoter region of Interferon gamma gene and also co-related this hypo-methylation with an increase in interferon gamma gene transcription in periodontitis biopsies as compared to healthy control gingival biopsies. Ishida K et al 2012,16 demonstrated that there was hypomethylation in the promoter region of IL-6 gene and co-related this with increased levels of interleukin 6 in peripheral blood of individuals of patients with chronic periodontitis. Zhang S et al 2013,17 evaluated the methylation status of the TNF alpha promoter at -161, -163 regions in the gingival biopsies of chronic periodontitis, healthy controls and reported a higher promoter region methylation (-163 region) in severe periodontitis patients as compared to controls. A more recent study,18 evaluating the TNF alpha gene promoter methylation in subjects with chronic periodontitis and rheumatoid arthritis revealed the chronic periodontitis subjects showed a significantly higher methylation rate and frequency at – 72 bp than the healthy gingiva group. Asaad F et al 2017,19 evaluated the DNA methylation status of inflammatory genes following treatment of chronic periodontitis. The authors reported periodontal therapy reduces the DNA methylation status of inflammatory gene for COX-2 in patients with periodontal disease, however DNA methylation levels of TNF-α, IFN-γ and LINE-1 remained unchanged in gingival tissue samples from periodontitis sites despite therapy.
Till date, there is no existing data associating methylation status of the promoter region (-239, -245) of the TNF alpha gene and chronic periodontitis. The objective of this study was to compare the methylation status of CpG islands in the promoter region of TNF alpha (-239, -245) gene in the peripheral blood among subjects with healthy gingiva and chronic periodontitis.
Methodology
The study was of a Case- control design. A total of 50 subjects were recruited for the study. The subjects were chosen from the patients visiting the Out-patient Department of Periodontology, Faculty of Dental Sciences, Sri Ramachandra University. Ethics approval was obtained from the Institutional Ethics Committee of Sri Ramachandra Institute of Higher Education and Research.
The case and control subjects were race- matched and were chosen within an age range of 30-60 years based on specific inclusion and exclusion criteria and after obtaining informed consent. A thorough history was elicited and subjects were examined for periodontal parameters listed in the proforma.
Sample Size Calculation
Confidence Interval: 95 %
Power: 80 %.
Precision: 10 %
With the above parameters; sample size needed to demonstrate the difference between the groups is 25 per group. A total of 50 samples are needed.
Group I- Cases- Subjects with chronic periodontitis – 25.
Group II- Controls- Subjects with healthy gingiva – 25.
Inclusion criteria for Group I
Subjects with chronic periodontitis were selected based on subject should have at least 10 teeth present, and on clinical examination subject should have clinical attachment loss of more than 1 mm in at least of 30% of the sites examined,20 should not have any systemic disease.
Inclusion Criteria for Group II
Controls include subjects who have clinically healthy gingival as determined by absence of bleeding on probing, no clinical attachment loss, no mobility or furcation involvement, should be otherwise systemically healthy and with no previous history of periodontal disease.
Exclusion Criteria for Both Groups I and II
Subjects who give a history of tobacco usage in any form, individuals who have taken antibiotics for past 6 months, individuals who have taken analgesics for past one week, pregnant and lactating women, presence of any other systemic disease, individuals who had undergone previous periodontal treatment were excluded from the study.
Clinical parameters examined in the proforma include the Oral Hygiene index,21 Gingival index,22 Plaque index,23 Periodontal probing at six sites per tooth to determine probing depth and attachment loss.
Probing depth will be measured from the gingival margin to the base of the pocket/sulcus. The attachment loss will be measured based on the following criteria:
The base of pocket is above the Cemento-enamel junction (CEJ): The distance from the gingival margin to the CEJ is subtracted from the probing depth to get the attachment loss value.
The base of the pocket is at the CEJ: The probing depth is equal to attachment loss.
The base of the pocket is below the CEJ: The probing depth is added with the distance from the gingival margin to CEJ.
The study population selected for the study was similar in age and gender distribution for both controls (healthy gingiva) and chronic periodontitis groups. The chronic periodontitis subjects had higher – plaque index, gingival index scores and probing pocket depth and clinical attachment level scores as compared to the controls (healthy gingiva) Table 3.
6 ml of Peripheral blood was collected from each subject of the case and control group under aseptic conditions in EDTA coated vacutainers by venipuncture in the ante-cubital fossa. The blood samples collected will be stored at -20°C. DNA isolation from the blood samples were carried out using the kit (Genetix Biotech Asia Pvt Ltd, Nucleo-pore DNA Sure Blood Mini Kit ) as per manufacturer’s protocol. 25 μl of Proteinase K is pipetted into the bottom of a 1.5ml microcentrifuge tube and to it add up to 200 μl blood. 200 μl Buffer GB3 is added to the samples and the mixture is vortexed vigorously (15 s). The samples are incubated at 70°C for 10 – 15 min. 210 μl ethanol (96 – 100 %) is added to each sample and vortexed again. The mixture is pipette into the DNA Sure Blood Mini Column placed in a Collection Tube. Centrifuge 1 min at 11,000 x g. Collection Tube with flow through is discarded. The DNA Sure Blood Mini Column is placed into a fresh Collection Tube (2 ml) and 500 μl Buffer GBW is added. Centrifuge 1 min at 11,000 x g. Collection Tube with flow through is discarded. The DNA Sure Blood Mini Column is placed into a fresh Collection Tube (2 ml) and 600 μl Buffer GB5 is added. Centrifuge 1 min at 11,000 x g. Flow-through is discarded and Collection Tube is reused. The DNA Sure Blood Mini Column is placed back into the Collection Tube and centrifuged at 1 min at 11,000 x g. Place the DNA Sure Blood Mini Column in a 1.5 ml microcentrifuge tube and add 100 μl preheated Buffer GBE (70°C). Dispense buffer directly onto the silica membrane. Incubate at room temperature for 1 min. Centrifuge 1 min at 11,000 x g.
Quantitative and qualitative evaluation of DNA isolated from the two sample groups
The amount and purity of the isolated DNA was determined by using a spectrophotometer. The DNA concentration was obtained by taking reading at 260 nm. The ratio of readings at 260 nm: 280 nm were used to determine the DNA purity.
Bisulfite Modification and Methylation Specific PCR [MS-PCR]
The isolated DNA was subjected to bisulfite treatment using Epitect Bisulfite conversion kit, as per the manufacturer’s instructions. In brief, after thermal denaturation and sodium bisulfite DNA conversion was performed , the DNA was then applied to an EpiTect spin column where, using optimized buffers and a standard microcentrifuge, it was washed to remove all traces of sodium bisulfite and eluted. The bisulfite converted DNA was then stored at – 20°C. MS-PCR was performed for the bisulfite converted DNA using methylated and unmethylated primers for the -245 and – 239 regions of TNF alpha promoter (Cordero P et al 2011).24 The PCR cycling conditions and the sequences of the methyalted and unmethylated primers are listed in Table 1.
Table 1: Primer sequences and methylation PCR conditions.
| Gene | Primer Sequence | MS-PCR Cycling Conditions |
| TNF-Alpha Methylated | 5’-TTAGAAGATTTTTTTCGGAATC-3’
5’-TATCTCGATTTCTTCTCCATCG-3’ |
(95°C-10 min; 95°C-30 sec; 57°C-1min; 72°C- 1 min) x38; 72°C – 7min |
| TNF-Alpha Unmethylated | 5’-GGTTTAGAAGATTTTTTTTGGAATT-3’
5’-TCTATCTCAATTTCTTCTCCATCAC-3’ |
Analysis of the MS-PCR Product
The MS-PCR products were then analyzed by SYBR green Real-Time PCR using methylated and unmethylated primers. The primers were diluted to 10pmol concentration. The reaction was performed in a 96-well plate. 2µl of the bisulfite converted DNA was added in duplicates (one well for methylation and one for unmethylation) to the corresponding wells. About 8µl of the reaction mix containing 5µl of SYBR green master mix, 0.5µl of the primers (forward and reverse), and 2µl of sterile water were added to the appropriate wells. The reaction mixes were prepared separately for methylation and unmethylation. Controls wells, containing sterile water and primers, labeled as NTC were prepared for both methylation and unmethylation. Fig 1 is representative pictogram of the Real Time PCR for detection of the methylation in TNF alpha promoter. The real time PCR program for TNF alpha methylation is given in Table 2.
 |
Figure 1: A representative image of the Real Time PCR amplification graph showing the intensity of fluorescence in Y axis against the number of amplification cycles in X axis, for detection of the methylation in TNF alpha promoter.
|
Table 2: Real time PCR cycling conditions.
| Temperature | Time |
| 50°C | 2 min |
| 95°C | 10 min |
| 95°C | 15 sec |
| 50°C | 1 min |
| 45 cycles |
Statistical Analysis
The mean and standard deviation of the demographic variables were analyzed. The difference in the frequency of methylation status in the control and test groups have been assessed and represented.
Results
The analysis of the MSP product was done by real time PCR. Only the presence or absence of methylation in the -245 and -239 CpG of TNF alpha gene was assessed in all the samples. A variable pattern of methylation was observed in the promoter region of TNF alpha. Amongst the control subjects (n=25), 8% (2/25) demonstrated a complete methylation of the TNF promoter region, 72 % (18/25) demonstrated a partial methylation, and 20% (5/25) demonstrated un-methylated promoter region. In the periodontitis group (n=25), 20% (5/25) demonstrated a complete methylation, 48% (12/25) demonstrated partial methylation and 32 % (8/25) demonstrated unmethylated promoter region. The mean Ct values for subjects showing methylation and lack of methylation in both control and periodontitis group are summarized in Table 4.
Table 3: Demographic data of the study subjects.
| Demographic variables | Healthy gingiva Group 1 |
Chronic Periodontitis Group 2 |
| Total no. of subjects | 25 | 25 |
| Males | 9 | 14 |
| Females | 16 | 11 |
| Age in years (mean ± SD) | 32.44 ± 3.20 | 36.45 ± 5.45 |
| Plaque Index (mean) | 0.95 ± 0.20 | 2.25± 0.75 |
| Gingival Index (mean) | 0.45 ± 0.15 | 2.35 ± 0.65 |
| Probing Pocket depth (PPD) (mm) | 1.7 ± 0.30 | 5.3 ± 0.65 |
| Clinical attachment Level (CAL) (mm) | 0 ± 0 | 4.5 ± 0.75 |
Table 4: Mean Ct values of subjects with complete methylation and lack of methylation in the TNF promoter (-239, -245) of control and periodontitis group.
| S.No | Methylation Status | Control (Mean Ct Value) | Periodontitis (Mean Ct Value) |
| 1. | Unmethylated | 25.15 | 25.45 |
| 2. | Methylated | 24.36 | 28.09 |
The mean Ct value for the methylation of control group was 24.36 and in the periodontitis group the mean Ct value was found to be 28.09. The higher the Ct value, the lower is the amount of methylation observed. The periodontitis group had a higher mean Ct value as compared to the control group; indicating a lower amount of methylation in the promoter region of the periodontitis group as compared to the control group. The data about the methylation patterns are summarized in Fig 2.
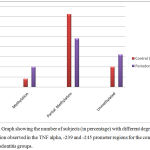 |
Figure 2: Graph showing the number of subjects (in percentage) with different degrees of methylation observed in the TNF alpha, 239 and -245 promoter regions for the control and periodontitis groups.
|
Discussion
The human genome serves as a blueprint for the structural and functional components of the human body. Alterations in the blueprint (Mutations) can lead to major genetic disordes affecting various organ systems of the human body as observed in the case of Turner’s syndrome.25 However, minor changes (polymorphisms) can contribute to an increased disease susceptibility of an individual to various acquired diseases of the human body. The genetic polymorphisms can contribute to an alteration in the structure, quantity, function of a protein for which the genes code. Genetic polymorphisms have been proposed to play a major role in Polygenic disorders. One such polygenic disorder is chronic periodontitis. Although, chronic periodontitis is primarily initiated by the bacterial plaque accumulation on the tooth surfaces, the progression of the disease is regulated by several factors such as systemic disease, environmental factors and genetic factors.
A landmark study performed by Loe H et al 1986,26 on Sri Lankan tea laborers, established the platform for the hypothesis “Individuals have genetic susceptibility for periodontitis”. The authors in this study evaluated the periodontal status and accumulation of dental calculus in subjects participating in this study. They observed that, in some subjects, although there was considerable amount of calculus accumulation, they did not show any attachment loss as compared to others who had lesser amount of calculus accumulation but also demonstrated attachment loss. These findings led the authors to hypothesize that there existed a genetic susceptibility to periodontal disease.
Later research, has evaluated the genetic susceptibility by studying single nucleotide polymorphisms in a variety of candidate genes, which were chosen based on their role in chronic periodontitis pathogenesis. The candidate genes include those coding for cytokines, immune receptors, host derived mediators.27 Two of the pro- inflammatory cytokines, which play a major role in periodontitis pathogenesis are Interleukin 1 and Tumor necrosis factor alpha. Tumor necrosis factor alpha is a member of the superfamily of ligand/receptor proteins called the tumor necrosis factor/tumor necrosis factor receptor superfamily proteins (TNF/TNFR SFP).28 It has a pro-inflammatory role and plays an important role in osteoclastogenesis and alveolar bone loss observed in periodontitis.29,30 Studies have evaluated the role of SNP ‘s in the TNF gene in periodontitis pathogenesis.31,32
In recent times, a paradigm shift has occurred in the concept of genetic susceptibility to disease. The current focus is on epigenetic alterations in the regulatory elements of gene expression such as promoter methylation, chromatin re-modelling (histone acetylation) and their potential role in periodontal disease. Recent studies have been done assessing the epigenetic changes in genes coding for inflammatory mediators such as IL-8, COX-2, and IFN Gamma. Hypomethylation of IL-8 gene promoter,13 hypomethylation of IFN gamma gene promoter,15 hypermethylation of the PTGS214 gene with corresponding downregulation of COX-2 levels14 and hypomethylation of the IL-6 gene16 have been reported in chronic periodontitis subjects as compared to control subjects with healthy gingiva.
In our study, we have assessed the methylation status of the -239 and -245 region of the TNF alpha gene in individuals with healthy gingiva and patients with chronic periodontitis.
Our study results demonstrated a variable pattern of methylation among subjects of the control and chronic periodontitis group. A lower level of TNF promoter methylation was observed in the periodontitis group as compared to the controls. This observation appears to support a de-methylation of the TNF alpha promoter in the periodontitis subjects.
Our study results are similar to the observations of Viana MB et al 2011,33 wherein the authors reported a variable pattern of methylation (total methylation, partial methylation, un-methylation) in the promoter of IFN gamma gene in gingival biopsies of periodontitis patients. Recent studies have reported hypermethylation of the promoter sites in gingival tissues from sites with chronic periodontitis.17,18,19 The authors reported a significantly higher level of methylation in biopsies of severe periodontitis cases as compared to healthy gingiva. The results of our study differ from the observations of the above mentioned study. This may be due to the different region of the TNF alpha promoter examined (-239 and -245) in our study. Further work to analyze the transcription levels of TNF alpha and correlating the transcription levels with methylation status of the promoter region of the TNF alpha gene needs to be carried out.
Acknowledgements
The authors wish to thank, Dr Mohanapriya of the Central Research Facility, Sri Ramachandra Institute of Higher Education and Research for the technical support for the study.
Conflict of Interest
The authors wish to report there is no conflict of interest with regards to the design and conduct of the study.
References
- Darveau RP, Tanner A, Page RC. The microbial challenge in periodontitis. Periodontol 2000. 1997 Jun;14:12-32.
- Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS.Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontol 2000. 1997 Jun;14:216-48.
- Kinane DF, Shiba H, Hart TC. The genetic basis of periodontitis.Periodontol 2000. 2005;39:91-117.
- Takashiba S, Naruishi K. Gene polymorphisms in periodontal health and disease.Periodontol 2000. 2006;40:94-106.
- Birkedal-Hansen H. Role of cytokines and inflammatory mediators in tissue destruction.J Periodontal Res. 1993 Nov;28(6 Pt 2):500-10.
- Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature 2007; 447:433-40.
- Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature 2004; 429: 457-63.
- Sharp AJ, Stathaki E, Migliavacca E, Brahmachary M, Montgomery SB, Dupre Y, et al. DNA methylation profiles of human active and inactive X chromosomes. Genome Res. Oct 2011; 21(10): 1592–1600.
- Das PM, Singal R. DNA methylation and cancer.J Clin Oncol. 2004 Nov 15;22(22):4632-42.
- Ventham NT, Kennedy NA, Nimmo ER, Satsangi J. Beyond gene discovery in inflammatory bowel disease: the emerging role of epigenetics. Gastroenterology. 2013 Aug;145(2):293-308.
- Lee HS, Herceg Z. The epigenome and cancer prevention: A complex story of dietary supplementation. Cancer Lett. 2014 Jan 28;342(2):275-84
- Wilson GA. Epigenetic regulation of gene expression in the inflammatory response and relevance to common diseases. J Periodontol 2008; 79: 1514-1519.
- Oliveira NF, Damm GR, Andia DC, Salmon C, Nociti FH Jr, Line SR, de Souza AP. DNA methylation status of the IL8 gene promoter in oral cells of smokers and non-smokers with chronic periodontitis. J Clin Periodontol. 2009 Sep;36(9):719-25.
- Zhang S, Barros SP, Niculescu MD, Moretti AJ, Preisser JS, Offenbacher S. Alteration of PTGS2 promoter methylation in chronic periodontitis. J Dent Res. 2010 Feb;89(2):133-7.
- Zhang S, Crivello A, Offenbacher S, Moretti A, Paquette DW, Barros SP. Interferon-gamma promoter hypomethylation and increased expression in chronic periodontitis. J Clin Periodontol. 2010 Nov;37(11):953-61.
- Ishida K, Kobayashi T, Ito S, Komatsu Y, Yokoyama T, Okada M, Abe A, Murasawa A, Yoshie H. Interleukin-6 gene promoter methylation in rheumatoid arthritis and chronic periodontitis. J Periodontol. 2012 Jul;83(7):917-25.
- Zhang S, Barros SP, Moretti AJ, Yu N, Zhou J, Preisser JS, Niculescu MD, Offenbacher S. Epigenetic regulation of TNFA expression in periodontal disease. J Periodontol. 2013 Nov;84(11):1606-16.
- Kojima A, Kobayashi T, Ito S, Murasawa A, Nakazono K, Yoshie H. Tumor necrosis factor-alpha gene promoter methylation in Japanese adults with chronic periodontitis and rheumatoid arthritis. J Periodontal Res. 2016 Jun;51(3):350-8.
- Asa’ad F, Bollati V, Pagni G, et al. Evaluation of DNA methylation of inflammatory genes following treatment of chronic periodontitis: A pilot case-control study. J Clin Periodontol. 2017 Sep;44(9):905-914.
- Armitage GC. Development of a classification system for periodontal disease and conditions. Ann Periodontol 1999;4:1-6.
- Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc. 1964 ; 68:7-13.
- Loe H. The Gingival index, the Plaque index and the Retention index system. J Periodontol 1967; 38: 610-16.
- Silness J and Loe H. Periodontal disease in pregnancy. II Corelation between oral hygiene and periodontal conditions. Acta Odontol Scand. 1964; 22: 112-35.
- Cordero P, Campion J, Milagro FI, Goyenechea E, Steemburgo T, Javierre BM, Martinez JA. Leptin and TNF-alpha promoter methylation levels measured by MSP could predict the response to a low-calorie diet. J Physiol Biochem. 2011 Sep;67(3):463-70.
- Rao E, Weiss B, Fukami M, Rump A, Niesler B, Mertz A et al. Pseudoautosomal deletions encompassing a novel homeobox gene cause growth failure in idiopathic short stature and Turner syndrome. Nature Genetics 1997 16, 54 – 63 .
- Löe H, Anerud A, Boysen H, Morrison E. Natural history of periodontal disease in man. Rapid, moderate and no loss of attachment in Sri Lankan laborers 14 to 46 years of age. J Clin Periodontol. 1986 May;13(5):431-45.
- Genetic susceptibility to periodontitis. Laine ML, Crielaard W and Loos BG. Periodontology 2000 2012; 58(1): 37–68.
- Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med. 1996 Jun 27;334(26):1717-25.
- Graves DT, Cochran D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol. 2003 Mar;74(3):391-401.
- Galbraith GM, Steed RB, Sanders JJ, Pandey JP. Tumor necrosis factor alpha production by oral leukocytes: influence of tumor necrosis factor genotype. J Periodontol. 1998 Apr;69(4):428-
- Schulz S, Machulla HK, Altermann W, Klapproth J, Zimmermann U, Gläser C et al. Genetic markers of tumour necrosis factor alpha in aggressive and chronic periodontitis. J Clin Periodontol. 2008 Jun;35(6):493-500.
- Moreira PR, Costa JE, Gomez RS, Gollob KJ, Dutra WO. TNFA and IL10 gene polymorphisms are not associated with periodontitis in Brazilian. Open Dent J 2009 Sep 7;3:184-90.
- Viana MB, Cardoso FP, Diniz MG, Costa FO, da Costa JE, Gomez RS et al. Methylation pattern of IFN-γ and IL-10 genes in periodontal tissues. Immunobiology. 2011 Aug;216(8):936-41.








