Nurhidayah Ab-Rahim*1 , Wan Ismahanisa Ismail1
, Wan Ismahanisa Ismail1 , Muhammad Nabil Fikri Roslan1
, Muhammad Nabil Fikri Roslan1 , Mohd Hafiz Mail2
, Mohd Hafiz Mail2 , Roz Azinur Che Lamin3
, Roz Azinur Che Lamin3 and Salmah Ismail4
and Salmah Ismail4
1Fakulti Sains Kesihatan, Universiti Teknologi MARA, Cawangan Pulau Pinang (Kampus Bertam), Malaysia.
2Malaysian Institute Pharmaceuticals and Nutraceuticals (IPHARM), NIBM, Pulau Pinang, Malaysia.
3Fakulti Farmasi, Universiti Teknologi MARA, Cawangan Pulau Pinang (Kampus Bertam), Malaysia.
4Institute of Biological Sciences, Faculty of Science, University of Malaya, Kuala Lumpur.
Corresponding author email: hidayahr@uitm.edu.my
DOI : https://dx.doi.org/10.13005/bpj/1710
Abstract
The rapid emergence of resistance bacteria toward various antibiotics may associate with higher medical cost and increase mortality rate. Hoya diversifolia was used to cure skin diseases and alleviate rheumatism pain. The aim of this study is to evaluate in vitro antibacterial properties of H. diversifolia ethanolic leaves extract against several Gram positive and Gram negative bacteria. The antibacterial study was determined based on pattern of inhibition zones using disc diffusion assay and also minimal inhibitory concentration (MIC). It is shown that the extract can inhibit the growth of methicillin-resistant Staphylococcus aureus, Escherichia coli and Bacillus cereus. The lowest MIC values of extract were 25 mg/mL for MRSA and E. coli as well as 100 mg/mL for B. cereus at 24 and 48 hours of incubation period. The plant had potential to act as antibacterial agent that can be applied in pharmaceutical and cosmetic fields.
Keywords
Antibacterial; Disc Diffusion; Hoya Diversifolia; Minimum Inhibitory Concentration
Download this article as:| Copy the following to cite this article: Ab-Rahim N, Ismail W. I, Roslan M. N. F, Mail M. H, Lamin R. A. C, Ismail S. Antibacterial activity of Hoya diversifolia ethanolic leaves extract. Biomed Pharmacol J 2019;12(2). |
| Copy the following to cite this URL: Ab-Rahim N, Ismail W. I, Roslan M. N. F, Mail M. H, Lamin R. A. C, Ismail S. Antibacterial Activity of Hoya Diversifolia Ethanolic Leaves Extract. Biomed Pharmacol J 2019;12(2). Available from: https://bit.ly/2IOPnfX |
Introduction
The utilization of plants as traditional medicines has been recognized a long ago to cure various diseases and infections. It is estimated that about 100,000 plant species have been proved for their medicinal values. In 2007, WHO stated that 25% of available drugs are originated from plants used in folk medicine since the plant-derived compounds show satisfying tolerance and acceptance among patients and turn into a reliable source of antimicrobial compounds.1 From 1981 to 2006, there are about 69% of 109 new antibacterial drugs are derived from natural products.2
Antimicrobial resistance led to global medical burden due to the current and potential impact on worldwide population health as the rate of mortality is increasing throughout the years.3 Furthermore, 39% of all resistant infections were originated from genetically mutated bacteria that are refused to last-line antibiotics, making them very challenging or impossible to treat.4 The degree of antibiotic-resistant infections is strongly connected to the person’s intolerance towards the antibiotic consumption. Ultimately, bacteria being able to inherit multiple resistance traits after a series of genetic mutation and becomes stronger resistance to the multiple classes of antibiotics.5
Hoya diversifolia is widespread in the areas of Indonesia, Malaysia, Thailand, Cambodia and Vietnam. The fleshy leaves produce white latex which is rich in triterpenyl cinnamate components.6 The tubular flowers with 5 lobes and tabular stigma appear as round or ball-like shaped.7 It is applied to relieve from the pain of rheumatism and as anti-nematodal agent against Bursaphelenchus xylophilus.8 In addition, this plant has also been used to cure skin diseases such as eczema, abscess, acne, boils, scabies, itch, infections, dermatitis, rash, sores, scar and warts.9 The present study was carried out to determine the antibacterial activity of the ethanolic leaves extract of H. diversifolia against methicillin-resistant Staphylococcus aureus (MRSA), Escherichia coli (E. coli) and Bacillus cereus (B. cereus).
Materials and Methods
Materials
Vancomycin antibiotic discs (30 µg; BD, USA); gentamicin antibiotic discs (10 µg; Oxoid, UK); ampicillin antibiotic discs (Oxoid, UK) and vancomycin antibiotic discs (Oxoid, UK); 96% ethanol (Merck, Germany); Columbia horse blood agar plates (Oxoid, UK); brain heart infusion (Oxoid, UK); Mueller-Hinton broth (Oxoid, UK); Mueller-Hinton agar (Oxoid, UK).
Collection of plant specimen and preparation of extract
Fresh H. diversifolia leaves were collected from Herbarium of Rimba Ilmu, Institute of Science Biology, University of Malaya, Kuala Lumpur. The leaves were washed using distilled water and dried in oven at 50°C for five days. The dried leaves were grinded using electrical blender. Hundred grams of the powdered leaves were soaked in 500 mL of 96% ethanol in conical flask for 3 days. After 3 days, the mixture was filtered using a fine muslin cloth and followed by filter paper (Whatman No. 1). The filtrate was evaporated under reduced pressure in Eyela rotary evaporator (Sigma-Aldrich, USA). The ready crude extract was kept in -20°C freezes until use.
Bacterial Culture
Three different species of bacteria were used in this study includes Escherichia coli ATCC25922, Bacillus cereus ATCC11778 and methicillin-resistant Staphylococcus aureus (MRSA). All bacteria were cultured and sub cultured again for purity on Columbia horse blood agar plates. The bacteria isolates were then maintained in brain heart infusion (BHI) agar slants at 4°C.
Antibacterial activity by disc diffusion assay
The Kirby-Bauer disc diffusion assay were carried out based on recommendations given by the Clinical Laboratory Standard Institute (CLSI).10,11 20 µL of different extract concentrations (200, 150, 100 and 50 mg/mL) were impregnated into 6 mm in diameter sterile, blank discs. Ethanol-loaded discs were then used as negative control. All discs were ensured to fully dried before being applied on the bacterial lawn. The positive controls are Gentamicin antibiotic disc (10 µg; Oxoid, UK) for B. cereus; ampicillin antibiotic disc for E. coli; vancomycin antibiotic disc for MRSA. The bacterial turbidity was adjusted with spectrophotometer to within an absorbance range of 0.8 to 0.13 at 625 nm with sterile broth, which equivalent to a 0.5 McFarland standard with a cell count of approximately 1.5 x 108 CFU/mL.11 The inoculums were used within 15 minutes of standardization as delays may change the inoculums size. The standardized bacterial inoculums were streaked over the entire surface of 90 mm Mueller-Hinton agar plate with sterile cotton swab three times, rotating the agar plate about 60 degree each time to ensure that the entire agar surface was covered with the inoculums. The inoculated plates were left with the lids ajar to allow excess moisture to dry for several minutes. The antibiotic discs were applied to the agar using sterile forceps and were pressed gently to ensure uniform contact. The discs were placed 6 discs per plate (positive and negative control disc included) equidistantly to avoid the overlapping of zones of inhibition. The plates were incubated in inverted position at 37°C overnight. Zones of inhibition were observed visually the next day. If present, their diameters were measured to the nearest whole millimeter with a ruler against a dark, non-reflective background. The assay was carried out in triplicates and the mean diameters of zone were calculated.
Determination of minimum inhibitory concentration (MIC) at 24 and 48 hours of bacterial incubation
Plates showing zones of inhibition from the disc diffusion assay were further tested to determine MIC values by broth macrodilution method as according to recommendations by CLSI.10,11 For ethanolic extract, 400 mg of extract was dissolved in 2 mL of sterile Mueller-Hinton broth (MHB) rather than the original solvent to obtain final concentration of 200 mg/mL. The stock solutions prepared (400 mg/mL) were serially diluted with sterile MHB to give concentrations of 200, 100, 50, 25, 12.5, 6.25, 3.125, 1.563, 0.781 and 0.391 mg/mL. The concentrations were prepared to a volume of 0.5 mL in separate microcentrifuge tubes (Eppendorf) at double the intended concentration so that addition of equal volumes of bacterial inoculums in later steps results in the desired final concentration in each tube. Bacterial inoculums were prepared with Mueller-Hinton broth and standardized to a 0.5 MacFarland standard (1.5 x 108 CFU/mL). The suspension was diluted 1:100 with sterile broth to obtain a cell count of approximately 106 CFU/mL. Then 0.5 mL of standardized bacterial suspension was added to the tubes containing the previously prepared 0.5 mL of diluted extract, thus resulting in a recommended final cell count of about 5 x 105 CFU/mL. A tube containing inoculated broth and extract solvent (sterile MHB) but without the leaf extract was served as the positive control. A tube containing broth alone (non-inoculated) and extract solvent was served as the negative control. All tubes were incubated overnight at 37ºC. The turbidity of the solution in each tube was observed the next day for indication of bacterial growth. The lowest concentration of extract dilution showing no visible growth (clear) was recorded as the MIC value. The tubes were further incubated for another 24 hours and were observed after 48 hours incubation for the absence or growth of bacteria (cloudy). The MIC value after 48 hours was recorded. MIC is defined as the lowest concentration where no visible turbidity is observed in the test tube.
Statistical Analysis
The results were expressed as mean ± SEM. Statistical analysis of the data were carried out using Student’s t-test and the results were considered significant as p<0.05.
Results and Discussion
Three different species of Gram positive and Gram negative bacteria were selected in this study includes E. coli ATCC25922, B. cereus ATCC11778 and methicillin-resistant S. aureus (MRSA). Ethanolic crude extract of H. diversifolia leaves showed antibacterial effect against all tested strains such as MRSA, E. coli and B. cereus with average inhibition zones based on concentrations of extract (Figure 1). The crude extract exhibited strong antibacterial activity against MRSA with the average inhibition zones from 11.83 to 14 mm. Lower antibacterial activity of the crude extract was indicated against E. coli with the average inhibition zones is 8 mm. It is suggested that antibacterial activity is rely on concentration of extract as increase the concentration of extract will increase the antibacterial activity (Table 1). The negative control showed no zones of inhibition and all positive controls against respective bacteria showed inhibition zones within susceptible range.
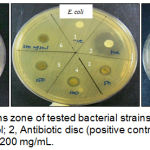 |
Figure 1: Growth inhibitions zone of tested bacterial strains by H. diversifolia leaves extract. 1, negative control; 2, Antibiotic disc (positive control); 3, 50 mg/mL, 4, 100 mg/mL, 5, 150 mg/mL, 6, 200 mg/mL.
|
H. diversifolia crude leaves extract showed antibacterial activity against MRSA, E. coli and B. cereus. MRSA and E. coli is main causal agent of serious skin and mucosal surface infection. Furthermore, MRSA is responsible for hospital-acquired infections.13 B. cereus is the most frequent agent of food poisoning outbreaks which can produce toxins that cause either emetic or diarrheal syndromes.14 Our study found that average inhibition zones up to 14 mm could be seen in the MRSA growth plate as the strongest inhibition than E. coli and B. cereus.
Sensitivity of test strains was observed in decreasing order: MRSA > B. cereus > E. coli. H. diversifolia crude leaves extract exhibited moderate antimicrobial activity against MRSA but least sensitive to E. coli as due to the composition variations in the outer membrane of cell wall. The outer membrane of cell wall in Gram negative bacteria is composed of lipopolysaccharide which play a role as a barrier to many substances including antibiotics.15 Moreover, the metabolism, nature and resistance to antibiotics may lead to non-susceptible of Gram negative bacteria towards crude leaves extract.13 This extract might have different modes of action on growth inhibitions of tested bacterial strains that gave different sensitivity.
Table 1: Mean diameter of growth inhibition zone by H. diversifolia leaves extract at concentrations of 50, 100, 150 and 200 mg/mL against tested bacterial strains.
| Bacterial strains | Inhibition zones (mm) | ||||
| Concentration of extract (mg/mL) | |||||
| 50 | 100 | 150 | 200 | Antibiotic disc | |
| MRSA | 11.8 ± 0.17 | 11.8 ± 0.0 | 13.0 ± 0.13 | 14.0 ± 0.0 | 17.67 ± 0.33 |
| E. coli | 8.0 ± 0.0 | 8.0 ± 0.0 | 8.0 ± 0.0 | 8.0 ± 0.0 | 18.33 ± 0.67 |
| B. cereus | 8.0 ± 0.0 | 9.3 ± 0.61 | 9.4 ± 0.0 | 9.8 ± 0.0 | 20.67 ± 0.33 |
| Data are means of three replicates (n = 3) ± SEM. Values greater than 6 mm indicated some activity | |||||
The lowest MIC value was 25 mg/mL against MRSA and E. coli whereas MIC value for B. cereus was 100 mg/mL. At this MIC point, this plant extract being able to work as bacteriostatic agent that can slow or stop the bacterial growth, mainly by interuption on protein synthesis. Ultimately, this mechanism can allow immune system to easily eradicate infectious agents from the host body.16 Study reported by Rahayu, Saputra and Setiawan (2017) stated that the same genus of Hoya which known as Hoya carnosa leaves showed strong inhibition activity against S. aureus and P. aeruginosa.17 Moreover, Hoya parasitica extract also showed antibacterial activity towards S. aureus, E. coli, Proteus spp, Hafnia spp., Enterococci spp., and Shigella spp.18 It is believed that Hoya species have potent antibacterial properties although there are no reported much on the biological activity of this species.
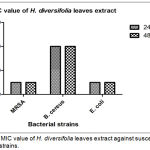 |
Figure 2: MIC value of H. diversifolia leaves extract against susceptible bacterial strains.
|
The value was expressed as the mean + SEM of experiment performed in three replicates (n=3).
It is assumed that high level of the MIC’s of the extract may correlated with some antagonistic agents that promote the growth of bacteria, therefore high amount of extract are required to inhibit the bacterial growth.12
Ethanol was chosen as a good solvent because of the stronger capacity of ethanol to extract out more active compounds from plant. At the same time, the ethanol extraction can enhance the presence of saponins, alkaloids, tannins and anthraquinones in the extracts.19 It is reported that H. diversifolia contains 81.7% triterpene cinnamates and 11.1% free trepenoids.20 Additionally, the leaves of H. diversifolia contain squalene, β-sitosterol, β-amyrin cinnamate and a mixture of α-amyrin, β-amyrin, and lupeol in a 4:2:1 ratio and saturated hydrocarbons.21 It is needed to perform the bioautography of H. diversifolia crude leaves extract in order to identify the specific antibacterial active compound (s); unfortunately, we were unable to provide the data due to limited facilities and equipment.
Conclusion
Crude leaves extract of Hoya diversifolia, which exhibited to be potentially effective as antibacterial agent against MRSA, S. aureus and B. cereus. This property was confirmed by the presence of inhibition zones in disc diffusion assay and minimal inhibitory concentration. Our finding provides new exploration on the biological effectiveness of Hoya diversifolia crude leaves extract especially on the interaction with bacterial strains. However, further investigations are required to be performed for development of novel antibacterial agents and assessment on growth inhibition of wide spectrum of bacteria and fungi species.
Acknowledgements
The authors acknowledge the help of Mr. Zulkapli Ibrahim of Rimba Ilmu Botanic Garden, University of Malaya, Malaysia for kindly providing the plant leaves. This project was supported by Postgraduate Research Fund (PPP) grant from University of Malaya (PV042/2011A).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Górniak, I., Bartoszewski, R., & Króliczewski, J. (2018). Comprehensive review of antimicrobial activities of plant flavonoids. Phytochemistry Reviews, Vol. 18 (1), 241-272.
- Newman, D. J. (2008). Natural Products as Leads to Potential Drugs: An Old Process or the New Hope for Drug Discovery? Journal of Medicinal Chemistry, 51(9), 2589–2599.
- Naylor, N. R., Atun, R., Zhu, N., Kulasabanathan, K., Silva, S., Chatterjee, A., … Robotham, J. V. (2018). Estimating the burden of antimicrobial resistance: a systematic literature review. Antimicrobial Resistance & Infection Control, 7(1).
- Hofer, U. (2019). The cost of antimicrobial resistance. Nature Reviews Microbiology, 17(1), 3.
- Zaman, S. B., Hussain, M. A., Nye, R., Mehta, V., Mamun, K. T., & Hossain, N. (2017). A Review on Antibiotic Resistance: Alarm Bells are Ringing. Cureus, 9(6): e1403.
- Groeneveld, H. W., & Van Der Burg, B. (1984). Quantitative aspects of triterpene synthesis in the laticifers of Hoya diversifolia Bl. Plant Science Letters, 33(1), 81–91.
- Wiart, C. (2010). Medicinal Plants of the Asia-Pacific – Drugs for the Future? Medicinal Plants of the Asia-Pacific – Drugs for the Future? World Scientific Publishing Co. Pte. Ltd.
- Alen, Y., Nakajima, S., Nitoda, T., Baba, N., Kanzaki, H., & Kawazu, K. (2015). Antinematodal Activity of Some Tropical Rainforest Plants against the Pinewood Nematode, Bursaphelenchus xylophilus. Zeitschrift Für Naturforschung C, 55(3–4), 295–299.
- Mollik, M. A. H., Hossan, M. S. H., Paul, A. K., Taufiq-Ur-Rahman, M., Jahan, R., & Rahmatullah, M. (2010). A comparative analysis of medicinal plants used by folk medicinal healers in three districts of Bangladesh and inquiry as to mode of selection of medicinal plants. Ethnobotany Research and Applications, 8, 195–218.
- Hindler, J. F. & Jorgensen, J. H. 2007. Antimicrobial susceptibility testing: Procedures in antimicrobial susceptibility testing. In: Mahon, C. R., Lehman, D. C. & Manuselis, G. (eds.) Textbook of Diagnostic Microbiology. 3rd ed. China: Saunders Elsevier.
- Jorgensen, J. H. & Turnidge, J. D. 2007. Susceptibility test methods: Dilution and disk diffusion methods. In: Murray, P. R., Baron, E. J., Jorgensen, J. H., Landry, M. L. & Pfaller, M. A. (eds.) Manual of Clinical Microbiology. 9th ed. Washington, District of Columbia: ASM Press.
- Khurram, M., Khan, M. A., Hameed, A., Abbas, N., Qayum, A., & Inayat, H. (2009). Antibacterial activities of Dodonaea viscosa using contact bioautography technique. Molecules, 14(3), 1332–1341.
- Oskay, M., & Sari, D. (2007). Antimicrobial screening of some Turkish medicinal plants. Pharmaceutical Biology, 45(3), 176–181.
- Majed, R., Faille, C., Kallassy, M., & Gohar, M. (2016, July 7). Bacillus cereus Biofilms-same, only different. Frontiers in Microbiology. Frontiers Media S.A. 7(1054), 1-16.
- Mudzengi, C. P., Murwira, A., Tivapasic, M., Murungweni, C., Burumue, J. V., & Halimani, T. (2017). Antibacterial activity of aqueous and methanol extracts of selected species used in livestock health management. Pharmaceutical Biology, 55(1), 1054–1060.
- Bernatová, S., Samek, O., Pilát, Z., Šerý, M., Ježek, J., Jákl, P., … Ružicka, F. (2013). Following the mechanisms of bacteriostatic versus bactericidal action using raman spectroscopy. Molecules, 18(11), 13188–13199.
- Rahayu, M. L., Saputra, K. A. D., & Setiawan, E. P. (2017). Antibacterial Activity Extract Hoya Carnosa Leaves, Chloramphenicol 1% and Ciprofloxacin against Staphylococcus aureus and Pseudomonas aeruginosa that caused benign type Chronic Suppurative Otitis Media (Disc Diffusion Method). Biomedical and Pharmacology Journal, 10(3), 1427–1432.
- Reze, M. S. H., Mandal, C., Alam, K. A., Salam, A., Rahman, M. A., Amin, M. R., Huda, M.N., Ghost, N. C., Ali, M. R. & Ahmed, F. (2009). Phytochemical, Antibacterial and Antinociceptive Studies of Hoya parasitica. Journal of Pharmacology and Toxicology, 2(8), 753–756.







