Inès Christelle Chadon Alphonsine Assemian* , Abdelhakim Bouyahya, Nadia Dakka and Youssef Bakri
, Abdelhakim Bouyahya, Nadia Dakka and Youssef Bakri
Laboratory of Human Pathologies Biology, Department of Biology, Faculty of Sciences, and Genomic Center of Human Pathologies, Faculty of Medicine and Pharmacy, Mohammed V University, Rabat 10 000, Morocco.
Corresponding Author E-mail: ines_christelle@yahoo.fr
DOI : https://dx.doi.org/10.13005/bpj/1676
Abstract
Garcinia mangostana L. is medicinal plant. Its fruit, the mangosteen contains many bioactive xanthones. This study investigates the potential of organic leaf extracts of G. mangostana grown in Ivory Coast. We tested two organic leaf extracts: G. mangostana aqueous ethanolic leaf extract (ethanol: water, 80:20 v/v) (GMLE) and dichloromethane leaf extract (GMLD). We measured total phenolic and total Flavonoids. We analysed the in vitro anti-inflammatory, anti-radical and anti-proliferative activities of leaf extracts. Ethanol leaf extract showed a considerable amount of phenolic content (328.78±34.32 mg GAE/g) and moderate flavonoids content (43.60±1.48 mg QE/g), Dichloromethane extract had low values of phenolic (70.31±4.55 mg GAE/g) and flavonoids (8.49±0.69 mg QE/g). However, GMLD extract gave a significant anti-inflammatory activity (IC50=152.79±3.34 µg/mL), comparable to the standard drug diclofenac sodium (IC50=142.30±1.22 µg/mL), contrary to GMLE extract (IC50=652.33±12.23 µg/mL). The radical scavenging assay showed a very significant ability of ethanol leaf extract to reduce the DPPH radical (IC50=33.40±0.67 µg/mL) compared to references molecules such as Trolox (IC50=43.72±0.31 µg/mL) and acid ascorbic (IC50=27.20±0.17 µg/mL), dichloromethane extract results showed lowest activity (IC50 = 580.00±23.03 µg/mL). All the organic leaf extracts of G. mangostana had moderate anti-proliferative activity on L20B, RD and VS cell lines studied with IC50 values ranging from 110.89 ±4.82 µg/mL to 860.60±25.78 µg/mL). Our results prove the high potential of the G. mangostana leave extracts as anti-inflammatory and anti-oxidative stress drugs. However, further studies are to determine and validate all the medicinal properties of G. mangostana leaves extracts.
Keywords
Anti-Cancer; Anti-Inflammatory; Anti-Radical; Garcinia Mangostana; Leaf Extracts
Download this article as:| Copy the following to cite this article: Assemian I. C. C. A, Bouyahya A, Dakka N, Bakri Y. Garcinia Mangostana Leaf Extracts from Ivory Coast Possess Remarkable Antioxidant, Anti-Inflammatory, and Cytotoxicological Properties. Biomed Pharmacol J 2019;12(2). |
| Copy the following to cite this URL: Assemian I. C. C. A, Bouyahya A, Dakka N, Bakri Y. Garcinia Mangostana Leaf Extracts from Ivory Coast Possess Remarkable Antioxidant, Anti-Inflammatory, and Cytotoxicological Properties. Biomed Pharmacol J 2019;12(2). Available from: https://bit.ly/2J3znab |
Introduction
Therapeutics researches have, since the early 1990s, focused on finding and improving new synthetic molecules.1 Since then, there has been a renewed interest in traditional medicine, which was the starting point for many researches on traditional medicines but especially on the pharmacognosy of the traditional plants.2 G. mangostana, is a small evergreen tree with a pyramidal crown, having one of the tastiest fruits, the mangosteen.3 Traditional medicines use its fruit hulls (pericarp), rinds, leaves, bark, and fruit pulp for many health affections, including skin infections, wounds, and diarrhea.4 It contains many active phytochemical screened in several laboratory studies showing anti-oxidant, anti-bacterial, anti-cancer, antimalarial, anti-inflammatory and antidiabetic effects.5 However, while the pharmacology of the fruit of G. mangostana is well-established, at our knowledge, there is a lack of phytochemical and pharmacological studies on the other parts of the tree, especially the leaves. Therefore, the present study determine flavonoids and phenolic contents, anti-inflammatory, anti-radical and anti-cancer effects of G. mangostana leaf extracts grown in Ivory Coast.
Material and Methods
Plant Material and Preparation of Extracts
The leaves of G. mangostana were collected from Anyama, Ivory Coast (N5°29’59.148 W4°3’9.853), in September, 2017. Samples were identified by the Ivorian department of plant resources of National Center for Agricultural Research (CNRA). A herbarium has been deposited in the department to be inserted into the herbal collection, no references were needed. The leaves collected were dried under shade for two weeks.
The dried samples were powdered and weighed (100g) and were consecutively extracted with 400 ml of each organic solvent dichloromethane and aqueous ethanol (80/20; Alcohol/water) using Soxhlet and then filtered (Whatman 10347673 Quantitative-filter-paper, England). The filtrate obtained was concentrated in a rotary evaporator (HeidolphTyp VV 1- Germany) to obtain the crude extract respectively GMLD and GMLE stored at 4°C for further uses.
Total Flavonoid Content (TFC)
We used aluminium chloride (AlCl3) as classic colorimetric method for the determination of flavonoids with quercetin as standard solution.6 The experimental procedure is described here after: 1 ml of the extract (1mg/mL) diluted in methanol were mixed with 1 ml of 2% AlCl3. A concentration range of quercetin (20, 40, 60, 80 and 100 mg/ml) was submitted to the same protocol. After 40 min of incubation at room temperature, we measured the absorbance at 430 nm using a spectrophotometer UV-VIS (Jasco V-630, Deutschland). Flavonoid content was determined the following formula of quercetin equivalent (QE):
y = 0.0238 * x – 0.0367
(R²= 0.9998)
Where y is the absorbance; x Quercetin concentration (mg QE); R² the correlation coefficient. The result was expressed as the quercetin equivalent per gram of extract (mg of QE/g of extract). All samples were analyzed in triplicate.
Total Phenolic Content (TPC)
TPC of the plant leaf extract were estimated by the Folin-Ciocalteu assay.7 Briefly, the extract or standard solution of acid ascorbic were diluted with methanol. Different concentrations (20, 40, 60, 80 and 100 mg/ml) were tested and 100 µL of each concentration were taken with 500 µL of Folin-Ciocalteu reagent diluted 10x and 400 µL of Na2CO3 (7%). After 40 min of incubation we measured the absorbance at 760 nm. For comparison we used a blank sample. We determined TPC by a calibration curve:
y = 0.004 * x + 0.5835
R² = 0.9452
Where y is the absorbance, x is Gallic Acid concentration (mg GAE/g) of extract; R² the correlation coefficient. TPC expressed as gallic acid equivalent (GAE)
Anti-Inflammatory Activity
The anti-inflammatory activity of G. mangostana extracts was assessed by the inhibition of protein denaturation protocol as describe by Anoop et al.,8 with slight modifications. Diclofenac Sodium was used as reference drug. In different tubes we mixed 0.05 ml of various concentrations (31.25 to 1000 µg/ml), of extracts and the reference and 0.45 ml of bovine serum albumin (BSA) (5%), the pH was fixed at 6.3 with 1N hydrochloric acid. All extracts were incubated at 37°C for 20 min and then submitted to an increase of temperature (57°C) for 3 min. After 15min, we added 2.5 ml of phosphate buffer solution (PBS) (pH=6.3) into each reactional tube and we measured the absorbance at 614 nm with a spectrophotometer (Jasco V-630, Deutschland). The control test tubes contained distilled water and BSA, the product control test tubes contained distilled water and the extracts. The percentage of inhibition was estimated with the following equation:
% Inhibition = 100 – ((O.D test – O.D product control)/ O.D control) * 100)
The concentrations which gave 50% of inhibition (IC50) were calculated. All concentrations were tested three times and we used the average.
Antioxidant Activity
The anti-radical activity of different extracts of Garcinia mangostana was investigated by using the organic chemical compound 2.2-diphenyl-1-picryl-hydrazyl (DPPH) with Trolox and ascorbic acid as reference drugs for the positive control. The violet color of DPPH turns into yellow when it is reduced by an antioxidant molecules. The color intensity is inversely proportional to capacity of the antioxidant molecules present in the medium to give protons. The method used was described by Bouyahya et al.,9 The method protocol consisted in adding 1.8 ml of DPPH (0.004% in methanol) 1.8 ml to a concentration range of extracts (31.25–1 000 µg/ml). The reaction was incubated 30 min at room temperature and we read the absorbance at 517nm. The reaction tube of the control test did not contain extracts. The scavenging activity in percentage was estimated with the following equation:
Free radical scavenging (%) = ((O.D control – O.D test)/ OD control) × 100
The concentrations which gave 50% of inhibition (IC50) were estimated using the graph of free radical scavenging against extracts and standards concentrations. All concentrations were tested three times and we used the average.
Anti-Proliferative Activity
Cell Lines Identification and Culture
The anti-proliferative activity was tested against three reference cancerous cells: Vero cells: Monkey kidney (ATCC N°CCL-81), L20B cells: Rat Rhabdomyosarcoma (3T6Swiss albino, ATCC CCL96) and RD cells (Embryonic Rhabdomyosarcoma, ATCC N°CCL-136). These cell lines are commonly used in our laboratory because of their accessibility and they are easier for results exchanges, maintenance and cultivation. They are provided from the National Institute of Health of Morocco. We used the protocol described by Aneb et al.,10The culture of cells is done in DMEM culture media (Gibco). The incubation temperature is 37°C in a humid atmosphere enriched with carbon dioxide 5% (CO2).
Anti-Proliferative Activity Protocol
The anti-proliferative activity was estimated using the MTT (3-(4.5-dimethylthiazol-2-yl)-2.5-diphenyltetrazoliumbromide) test provided by (Promega, USA). Basically, MTT is a yellow dye which switch to purple blue formazan when it is reduced by mitochondrial dehydrogenase of living cells attesting of cell viability. The method consisted in adding 100 µl of growth medium containing 3-4 x 106 cells/mL of cells in microplates (96 wells) incubated 24h at 37°C in 5% CO2. Then, we treated each well with 100 µL of a concentration range of extracts 3.5 to 250 µg/ml. Microplates were incubated for 48h in the same conditions. The control wells did not contain extracts. After adding 20 µL of the MTT to each well, 3h were necessary for incubation and we ended the reaction with 100 µL of isopropanol (50%) and sodium dodecyl sulfate (SDS) (10%). After 30min, we read the absorbance at 550 nm using an ELISA plate reader. The cell inhibition percentage was estimated using the following equation:
Inhibition (%) = 100 × (O.D control – O. D test) /O.D control)
All the concentrations were tested three times. The concentrations which gave 50% of inhibition (IC50) were estimated using the graph of cell inhibition percentage against extracts concentrations. PBS and DMSO (1%) were used as negative control.
Statistical Analyses
The results of experiment were expressed as mean ± SD of three independent experiment. To compare the statistical significance between groups we used a Student t test. P≤ 0.05 implied a significant difference between groups or values.
Results
Total Flavonoid Content (TFC) and Total Phenolic Content (TPC)
TFC, estimated from a calibration curve of quercetin, showed that the concentration of flavonoid in the ethanol leaf extract GMLE of the plant is significantly high (43.60±1.48) comparing to the value (P < 0.05) of flavonoid content of the dichloromethane extract GMLD (8.49 ±0.69) QE mg/g extract. TPC of leaf organic extracts estimated using Acid Gallic as standard showed that the ethanol extract contains a considerable quantity of phenolic compounds with a value of (328.78±34.32) GAE mg/g despite of the dichloromethane extract (70.31±4.55) GAE mg/g (Table 1).
Table 1: Extraction efficiency, Total Flavonoids Content (TFC) and Total Phenolic Content (TPC)
| Extract | Yield (w: w %) | TFC (mg QE/g DW) | TPC (mg GAE/g DW) |
| GMLE | 26.72a | 43.60 ± 1.48a | 328.78 ± 34.32a |
| GMLD | 13.12a | 8.49 ± 0.69b | 70.31 ± 4.55b |
Significant differences (p < 0.05) marked with different letters; n =3
In Vitro Anti-Inflammatory Activity
The method we used to investigate the anti-inflammatory capacity of our extracts was the protocol of inhibition of thermally induced protein denaturation. For this purpose, various concentrations of leaf extracts (31.25 to 1000 µg/mL) were tested and compared with the reference drug Diclofenac Sodium. Results are shown in Graph 1. Results presented in the figure showed that GMLE extract inhibition capacity is low and barely exceed 50% at the concentration of 1000 µg/mL. GMLD anti-inflammatory activity is similar to the reference drug Diclofenac sodium, their activities are comparable at the different concentrations. The determination of IC50 provides a better insight into the ability of each extract to inhibit protein denaturation (Table 2). The values of the dichloromethane (IC50=152.79±3.34 µg/mL) and diclofenac (IC50=142.30±1.22 µg/mL) are significantly comparable (P < 0.05).
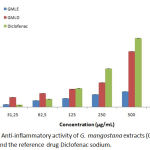 |
Graph 1: Anti-inflammatory activity of G. mangostana extracts (GMLE and GMLD ) and the reference drug Diclofenac sodium.
|
Table 2: Anti-inflammatory and Antioxidant activities, IC50 values, of G. mangostana organic leaf extracts.
| GMLE | GMLD | Ascorbic acid | Trolox | Diclofenac | |
| Anti-inflammatory inhibition IC50 (µg/ml) | 652.33±12.23b | 152.79±3.34a | – | – | 142.30±1.22a |
| Anti-radical capacity IC50 (µg/ml) | 33.40±0.67a | 580.00±23.02b | 27.20±0.17a | 43.72±0.31a | – |
Significant differences (p < 0.05) marked with different letters; n =3
Antiradical Activity
DPPH scavenging ability of G. mangostana organic leaf extracts are presented in Graph 2. The figure showed that each concentration of extract caused a DPPH reduction. The GMLD extract scavenging capacity is low at the concentration of 31.25 to 500 µg/mL but significant when the concentration of the extract is 1000 µg/mL. GMLE extract scavenging activity is important since the lowest concentration and increases subsequently till the highest concentration tested. However, graphic showed a plateau reach at 250 µg/mL, which corresponding probably to the saturation concentration.
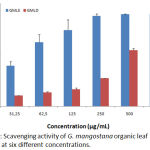 |
Graph 2: Scavenging activity of G. mangostana extracts at six different concentrations.
|
IC50 values of each extracts were determinate and compared to the reference molecules ascorbic acid and Trolox results are reported in Table 2. As confirmed by the table, GMLD extract anti-inflammatory capacity is low (IC50=580.00±23.02 µg/mL) comparing with Trolox (IC50=43.72±0.31µg/mL) and Acid ascorbic (IC50=27.20±0.17 µg/mL). While GMLE shows a significant anti-radical activity (IC50=33.40±0.67 µg/mL) sensibly equal to reference molecules.
Anti-Proliferative Activity
We attempted to evaluate the inhibition ability of our extracts against three cancer cell lines: RD, L20B and Vero cells. Like showed in graph 3, extracts presented mixed inhibitory capacity on the different cells. (IC50) values presented in table 3 permitted a comparison between the various effects of extracts. As summarised, the two extracts showed low anti-proliferative activities against the three cell lines that varies between the types of extract. However, both GMLE and GMLD tumour inhibition were more effective on Vero cells, their IC50 values were respectively 110.89±4.82 and 167.17±2.20 µg/ml. For L20B, the IC50 values of the same extracts were respectively 337.54±4.22 and 428.32± 4.26 µg/ml. The cytotoxicity activity against RD tumour cell lines were even less efficient with IC50 reaching amounts of 659.43±12.42 µg/mL and 860.60±25.78 µg/ml.
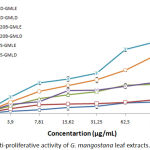 |
Graph 3: Anti-proliferative activity of G. mangostana leaf extracts.
|
Table 3: Anti-proliferative activity IC50 values of G. mangostana organic leaf extracts against Vero, L20B and RD cancerous cell lines.
| Anticancer activity IC50(µg/mL) | |||
| Extract | Vero | L20B | RD |
| GMLE | 110.89 ± 4.82a | 337.54 ± 4.22b | 659.43 ± 12.42c |
| GMLD | 167.17 ± 2.20a | 428.32 ± 4.26b | 860.60 ± 25.78c |
Significant differences (p < 0.05) marked with different letters; n =3.
Discussion
Nowadays, medicinal plants are more and more attractive because of the large number of bioactive molecules they contain which are part of their secondary metabolism also call specialized metabolism.11 Among the world pharmacopoeia, the Garcinia genus in the family of Clusiaceae is widely represented with about 250 species of trees found throughout the tropics.14 Part of them are important in local traditional medicine, the others are cultivated for their fruit or as ornamentals. G. mangostana is best known for its fruit, the mangosteen, which is the subject of many researches because of the large amount of xanthones and other bioactive molecules it contains.15 Several parts of the plant are used in traditional medicine, stem bark, roots, pericarp or all fruit.
They were studied in laboratory to determine the chemical composition of the different parts and their biological activity potential.16 However, leaves of G. mangostana are still less studied than the other parts of this medicinal plant. In this study, we proceed to the selection and identification of the plant leaves, followed by a solid-liquid Soxhlet extraction to obtain two organic extracts of leaves the dichloromethane extract (GMLD) and the ethanol extract (GMLE) and tested estimated their phenolic and flavonoid content, and determine their anti-inflammatory, anti-radical and anticancer activities. Results have shown that the ethanol leaf extract contains an important quantity of polyphenols (328.78±34.32mg GAE/g) and moderate quantities of flavonoids (43.60±1.48mg QE/g) five times more than the quantities found in the dichloromethane leaf extract. These results can be explained by the fact that ethanol and dichloromethane are different types of solvents. Ethanol is a polar solvent that allows interactions with ionic and polar solids and promotes their dissolution. It can dissolve ionic compounds such as chlorides and hydroxides.17 While, dichloromethane is a nonpolar solvent, it can dissolve molecular solids. It is a solvent extractor of organic compounds.18 Following this evidence, the biological composition and effect of GMLE and GMLD will presents many differences. Moreover, another study confirmed the important quantity of phenols in G. mangostana leaf extracts.19 However, the chemical composition of plants is highly subject to variations in its environment.20
In this study, we used the protein denaturation method to investigate the anti-inflammatory activity of leaf extracts of G. mangostana. The various extracts showed a dose dependent percentage of inhibition of protein denaturation. Dichloromethane extract had a significant anti-inflammatory activity comparable to the reference drug, according to results (P < 0.05), counter to ethanol extract which activity was quite weak, suggesting that the dichloromethane extract is rich in anti-inflammatory compound that can be further analysed with other method in vivo or in vitro for confirmation. To our knowledge, no other studies on G. Mangostana anti-inflammatory activity of leaf extracts were made. However, many in vivo and in vitro anti-inflammatory activity studies of the plant fruit hull, pericarp, and peel were carried out.21–23
For the antioxidant activity, our results showed a dose dependent percentage inhibition of DPPH reduction with remarkable results for the ethanol leaf extract, having a free radical scavenging activity more efficient than Trolox a reference drug. This activity is directly correlated with the high amount of phenolic and flavonoid content found in the ethanol leaf extract. Indeed, many studies described polyphenols and flavonoids as potentials antioxidant molecules having various reduction abilities.24 Experiments showed a plateau from the concentration of 250 µg/ml. That means that this concentration corresponds to the maximum amount of a substance that can be dissolved in the solvent at ambient temperature we used for the reaction. However, various factors can influence this parameter (the concentration of the solute, temperature, polarity and the solute or solvent).25 We noticed in this study that GMLE extract had a remarkable anti-radical activity, but a low anti-inflammatory activity, whereas GMLD extract had a low anti-radical activity but a remarkable anti-inflammatory activity. That suggests that the dichloromethane solvent extracts contains a huge quantity of molecules structurally different from polyphenols and flavonoids having anti-inflammatory activities may be terpenoids which anti-inflammatory activities was proven.26 Another study on G. mangostana leaf extract was carried out for antioxidant activity.19 In this study, the ethanol extract had a low anti-radical activity. These contradictory results can be explained by differences in extraction protocols, the difference of method used for investigation, but also a difference in leaf grows, collection and conservation.
Also, we tested the anticancer activity of G. mangostana organic leaf extract, GMLE and GMLD against Vero, L20B and RD cells. Our results showed that all extracts had moderate anticancer activities, even if Vero cells were more sensitive to them. This can be due to the low quantity of flavonoids found in both extracts. In fact, various researches demonstrated the anticancer potential of flavonoids.27 Studies on G. mangostana ethanol leaf extract against B16-F10 cancerous cell lines gave significant results as potential anticancer.28 Various factors cited previously in the analyses could justify the differences observed. A possible correlation between TPC and TFC and anti-radical activity of G. mangostana leaf extracts could be established. However, further chemical analyses must be attempted to determine the molecules inducing the anti-inflammatory activity. Moreover, all the parameters of this study depend on several factors such as the plant itself and culture condition, the used solvents and different methods.
Conclusion
The anti-inflammatory and antiradical activities of G. mangostana organic leaf extracts have shown very interesting results proving that the leaves of this plant are potential candidate to new traditional and conventional medicines. Though these remarkable activities have a solvent extract dependant action and their use must be modulated by a precise methodology calibration. Moreover, other analyses on anticancer activity are needed to certify their ability to repress some cancerous cell lines with a selective action.
Acknowledgement
We are grateful to the Moroccan Agency of Medicinal and Aromatic Plants (AMPAM) for its financial support.
Conflict of Interest
There is no conflict of interest.
Funding Source
None
Reference
- Pawe\lczyk A, Sowa-Kasprzak K, Olender D, Zaprutko L. Molecular consortia—Various structural and synthetic concepts for more effective therapeutics synthesis. Int J Mol Sci. 2018;19(4):1104.
- Orhan IE. Pharmacognosy: Science of natural products in drug discovery. BioImpacts BI. 2014;4(3):109.
- Cassileth B. Mangosteen (Garcinia mangostana). Oncol Williston Park N. août 2011;25(9):844.
- Jain SK, Srivastava S. Traditional uses of some Indian plants among islanders of the Indian Ocean. 2005;
- Chaverri JP, RODRIGUEZ NC, IBARRA MO, ROJAS JMP. Medicinal properties of mangosteen (Garcinia mangostana). 2019;
- Et-Touys A, Fellah H, Mniouil M, Bouyahya A, Dakka N, Abdennebi EH, et al. Screening of antioxidant, antibacterial and antileishmanial activities of Salvia officinalis L. extracts from Morocco. Br Microbiol Res J. 2016;16:1–10.
- Bouyahya A, Abrini J, El-Baabou A, Bakri Y, Dakka N. Determination of phenol content and antibacterial activity of five medicinal plants ethanolic extracts from North-West of Morocco. J Plant Pathol Microbiol. 2016;7(342):2.
- Anoop MV, Anees P, Naseeha Ismayil RK, Raheela V, Nadakkavail SS. Evaluation of in-vitro anti-inflammatory activity of Coreopsis lanceolata L. flower petals. 2015;
- Bouyahya A, Abrini J, Bakri Y, Dakka N. Screening phytochimique et évaluation de l’activité antioxydante et antibactérienne des extraits d’Origanum compactum. Phytothérapie. 2017;15(6):379–383.
- Aneb M, Talbaoui A, Bouyahya A, El Boury H, Amzazi S, Benjouad A, et al. In vitro cytotoxic effects and antibacterial activity of Moroccan medicinal plants Aristolochia longa and Lavandula multifida. Eur J Med Plants. 2016;16(2):1–13.
- Tissier A, Ziegler J, Vogt T. Specialized plant metabolites: diversity and biosynthesis. Ecol Biochem Environ Interspecies Interact. 2014;14–37.
- Chang H-H, Cohen T, Grad YH, Hanage WP, O’Brien TF, Lipsitch M. Origin and proliferation of multiple-drug resistance in bacterial pathogens. Microbiol Mol Biol Rev. 2015;79(1):101–116.
- Arnold C. The new danger of synthetic drugs. The Lancet. 2013;382(9886):15–16.
- Te-chato S. Floral and fruit morphology of some species in Garcinia spp. Songklanakarin J Sci Technol. 2007;29(2):245–252.
- Murthy HN, Dandin VS, Dalawai D, Park S-Y, Paek K-Y. Breeding of Garcinia spp. In: Advances in Plant Breeding Strategies: Fruits. Springer; 2018. p. 773–809.
- Gutierrez-Orozco F, Failla M. Biological activities and bioavailability of mangosteen xanthones: A critical review of the current evidence. Nutrients. 2013;5(8):3163–3183.
- Welton T. Solvents and sustainable chemistry. Proc R Soc Math Phys Eng Sci. 2015;471(2183):20150502.
- Levina II, Klimovich ON, Vinogradov DS, Podrugina TA, Bormotov DS, Kononikhin AS, et al. Dichloromethane as solvent and reagent: a case study of photoinduced reactions in mixed phosphonium-iodonium ylide. J Phys Org Chem. 2018;31(7):e3844.
- Chew Y-L, Lim Y-Y. Evaluation and Comparison of Antioxidant Activity of Leaves, Pericarps and Pulps of Three Garcinia Species in Malaysia. Free Radic Antioxid. 2018;8(2).
- Jamali CA, Kasrati A, Bekkouche K, Hassani L, Wohlmuth H, Leach D, et al. Phenological changes to the chemical composition and biological activity of the essential oil from Moroccan endemic thyme (Thymus maroccanus Ball). Ind Crops Prod. 2013;49:366–372.
- Chen L-G, Yang L-L, Wang C-C. Anti-inflammatory activity of mangostins from Garcinia mangostana. Food Chem Toxicol. 2008;46(2):688–693.
- Putri K, Darsono L, Mandalas H. Anti-inflammatory properties of mangosteen peel extract on the mice gingival inflammation healing process. Padjadjaran J Dent. 2017;29(3).
- Yang R, Li P, Li N, Zhang Q, Bai X, Wang L, et al. Xanthones from the Pericarp of Garcinia mangostana. 2017;22(5):683.
- Saxena M, Saxena J, Pradhan A. Flavonoids and phenolic acids as antioxidants in plants and human health. Int J Pharm Sci Rev Res. 2012;16(2):130–134.
- Lu JX, Bhimji S. Chemistry, Dissolution and Solubility. 2017;
- Souza MT de S, Almeida JRG da S, Araujo AA de S, Duarte MC, Gelain DP, Moreira JCF, et al. Structure–Activity Relationship of Terpenes with Anti-Inflammatory Profile – A Systematic Review. Basic Clin Pharmacol Toxicol. 2014;115(3):244‑
- Genoux E, Nicolle E, Boumendjel A. Flavonoids as anticancer agents: recent progress and state of the art? Curr Org Chem. 2011;15(15):2608–2615.
- Cunha BLA, França JP de, Moraes AA de FS, Chaves ALF, Gaiba S, Fontana R, et al. Evaluation of antimicrobial and antitumoral activity of Garcinia mangostana L. (mangosteen) grown in Southeast Brazil. Acta Cir Bras. 2014;29:21‑8.








