Parepalli Suresh1 , Alphienes Stanley Xavier*1
, Alphienes Stanley Xavier*1 , Karthik V. P1
, Karthik V. P1 and Punnagai K1
and Punnagai K1
Department of Pharmacology, Sri Ramachandra Institute of Higher Education and Research, Porur, Chennai - 600116, Tamil Nadu, India.
Corresponding Author E-mail: alphclinpharm@sriramachandra.edu.in
DOI : https://dx.doi.org/10.13005/bpj/1724
Abstract
Cissus quadrangularis has wide spectrum of benefits in medical conditions including bone disorders. Alcoholic extract of the plant displayed anticancer activity against cell lines derived from cervical, skin, colon, breast, as well as kidney cancers, and flavonoid fraction of the extract was found to be the active constituent for the activity. To evaluate the anticancer effects of Cissus quadrangularis leaf extract against MG63 human osteosarcoma cell line. MG63 cells were obtained from NCCS, Pune. The methanolic extract of Cissus quadrangularis was prepared and its anticancer activity was tested in cell lines using Mossman method of cytotoxicity assay. The cell viability of MG63 cells ranged between 29.65% and 73.59% at an extract concentration from 1000µg/ml to 7.8µg/ml. The IC50 of extract revealed by this cytotoxicity assay was around 100 µg/ml. This study showed anticancerous activity of C.quadrangularis leaf extract against MG63 cells, which can be further characterized by future studies and aid in treatment of bone tumors.
Keywords
Bone Tumor; Cancer; Cissus Quadrangularis; Methanolic Extract; MTT Assay
Download this article as:| Copy the following to cite this article: Suresh P, Xavier A. S, Karthik V. P, Punnagai K. Anticancer Activity of Cissus Quadrangularis L. Methanolic Extract Against MG63 Human Osteosarcoma Cells – An In-Vitro Evaluation using Cytotoxicity Assay. Biomed Pharmacol J 2019;12(2). |
| Copy the following to cite this URL: Suresh P, Xavier A. S, Karthik V. P, Punnagai K. Anticancer Activity of Cissus Quadrangularis L. Methanolic Extract Against MG63 Human Osteosarcoma Cells – An In-Vitro Evaluation using Cytotoxicity Assay. Biomed Pharmacol J 2019;12(2). Available from: https://bit.ly/2Xsi1K0 |
Introduction
Cancer is a disease with multistep and multiple factor pathogenesis, and a rising concern causing major health burden worldwide. According to the International Association of Cancer Registries (IACR – GLOBOCAN database), 12.7 million new cases of cancer, and 7.6 million deaths due to cancer were reported worldwide.1 The numbers increased to 14.1 million new cases and 8.2 million cancer deaths by 2012.2 The recently updated database in 2018 has reported new cancer cases of 18.1 million, and cancer deaths of 9.6 million, which proved the mounting burden of cancer incidence as well as mortality.3 In United States, bone carcinomas constitute 0.2% of all cancer conditions. Current treatment modalities for bone malignancies include surgeries, chemotherapy, radiotherapy, as well as immunomodulation which comprise of high mortality risk. This implicates an urgent need for new treatment strategies with fewer side effects to effectively combat malignant conditions.4
Natural products from plants are undeniably a vital resource to explore the possible lead compounds for the treatment of cancer. During the last five to six decades, plant products, metabolites, and their derivatives have been effectively introduced into the armamentarium to fight against cancer. More number of studies has been done to evaluate anticancer activity of plant samples, as well as plant extracts, and many of them successfully entered the market worldwide for treatment of cancer.5 Thus it is imperative to search for novel plant phytoconstituents which possess the ability to fight against cancer cells. Plant phytoconstituents rich in antioxidants were known to reduce cancer mortality, and increase life expectancy.6,7
Vinca alkaloids (vincristine, vinblastine), taxanes (paclitaxel), camptothecins (irinotecan, topotecan) and epipodophyllotoxins (etoposide) are the important plant derivatives which are being effectively used as anti-proliferative compounds in cancer chemotherapy.8 Phenolic compounds including phenolic acids, flavonoids, and tannins are the major constituents of the bioactive compounds derived from plants. Antioxidant properties of these compounds were well established in both benchside as well as bedside research studies. Potential anticancer activity of these compounds was attributed to their ability to cause cell cycle arrest, induce apoptosis, inhibit cell proliferation, and angiogenesis.9
Cissus quadrangularis. Linn is a perennial plant which belongs to Vitaceae (grape) family. It was reported to be native of India and Africa. The plant has been known by many names according to the geographical area. Some of the common names are Kandvel, Perandai, Asthisamdhani, Hadjod, Harbhanga, Varavalli etc. Almost all parts of the plant including stem, leaves, as well as roots are being used as medicine. The medicinal uses of Cissus quadrangularis were realized and well documented in native medicine including siddha, ayurvedha. The pharmacological properties were extensively studied in literature and of wide spectrum. The spectrum includes anti-inflammatory, analgesic, antitumor, antiosteoporotic, antibacterial, anticonvulsant, antipyretic, antifungal, antidiabetic, gastroprotective, and hepatoprotective.10,11,12 The phytochemical constituents of the plant have been characterized. The stem contains calcium, phosphorous which helps in bone formation. Some of the important constituents are amyrins (α,β), carotene, vitamin C, β-sitosterol, resveratrol, flavonoids such as quercetin, quadrangularins (A,B,C), and kaempferol.13 MG63 cells were derived from human osteosarcoma, which were well characterized, and able to provide better understanding to study anticancer activity against bone tumours.14 With this background we undertook an in-vitro study to investigate anticancer activity of C.quadrangularis methanolic extract against MG63 cells.
Materials and Methods
Plant Collection and Extract Preparation
Cissus quadrangularis plants were collected from localities around Chennai, Tamilnadu. The authentication was done by the Botanist Prof. V. Chelladurai, Central Council for Research in Ayurvedha and Siddha, Government of India. The aerial parts of the plants were dried up in shade and then the dried parts were powdered. The powder was subjected to methanolic extraction using Soxhlet apparatus.15
Cell Culture
Human MG63 cell lines were procured from the Cell repository of National Centre for Cell Sciences (NCCS), Pune, India. Dulbecco`s Modified Eagle Media (DMEM) was used for maintaining the cell line, which was supplemented with 10% Fetal Bovine Serum (FBS). Penicillin (100 U/ml), and streptomycin (100 μg/ml) were added to the medium to prevent bacterial contamination. The medium with cell lines was maintained in a humidified environment with 5% CO2 at 37°C.
Cytotoxicity Assay
The MG63 cells were placed in 24 well plates (1 X 105 cells per well) and incubated in 5% CO2 environment at 37°C. Cells (1 × 105/well) were placed in 24-well plates and incubated in 370C with 5% CO2 condition. Once the cells placed in wells reached confluence, the prepared concentrations of extract from 1000µg/ml to 7.8µg/ml were added and kept in incubator for 24 hours. Then the samples were removed from the well, and washed with phosphate-buffered saline (pH 7.4) or DMEM without serum. 0.5% 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl–tetrazolium bromide (MTT) was added to each well (100μl/well) and incubated for 4 hours. Then 1ml of dimethyl sulfoxide (DMSO) was added in all the wells to dissolve the formed formazan crystals. Each sample was placed in the cuvette; using DMSO as the blank the absorbance value at the wavelength of 570 nm was noted using Ultra-violet (UV) Spectrophotometer. The average absorbance values from three observations were taken. The observed values were tabulated, and the concentration required for 50% inhibition (IC50) was determined graphically. The percentage cell viability was calculated by determining the ratio between A570 of treated cells, and A570 of control cells, multiplied by 100. Cell control and sample control is included in each assay to compare the full cell viability assessments.16
Results
The MG63 cell viability with various concentrations of C.quadrangularis leaf extract was tabulated in table 1. The cell viability of MG63 cells ranged between 29.65% and 73.59% at extract concentrations of 1000 µg/ml and 7.8 µg/ml correspondingly. The methanolic extract of C.quadrangularis showed cytotoxicity against MG63 cells in concentration dependent manner [Table 1, Figure 1]. The calculated % cell viability was plotted against concentration of extract, and half maximal inhibitory concentration (IC50) was noted graphically. IC50 revealed by the assay was around 100 µg/ml at the dilution of 1:4. The cell viability and the cytological characteristics of MG63 cells can be observed in the microscopic images of cells treated with extract [Figure 2]. Exposure to increased concentration of the extract has grossly reduced the number of viable osteosarcoma cells, and their architecture was found to be disrupted.
Table 1: Cell viability of MG63 cells treated with Cissus quadrangularis methanolic extract.
| S.No | Extract concentration (μg/ml) | Dilution | Absorbance at 570 nm | % Cell viability |
| 1 | 1000 | Neat | 0.602 | 29.65 |
| 2 | 500 | 1:1 | 0.721 | 35.51 |
| 3 | 250 | 1:2 | 0.828 | 40.78 |
| 4 | 125 | 1:4 | 0.989 | 48.71 |
| 5 | 62.5 | 1:8 | 1.112 | 54.72 |
| 6 | 31.2 | 1:16 | 1.245 | 61.33 |
| 7 | 15.6 | 1:32 | 1.344 | 66.20 |
| 8 | 7.8 | 1:64 | 1.494 | 73.59 |
| 9 | Cell control | – | 2.030 | 100 |
O.D – Optical Density, % – percentage, Percentage cell viability at graded concentrations of plant extract.
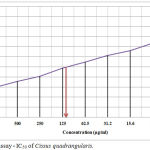 |
Figure 1: MTT assay – IC50 of Cissus quadrangularis methanolic extract
|
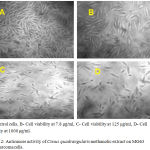 |
Figure 2: Anticancer activity of Cissus quadrangularis methanolic extract on MG63 osteosarcoma cells
|
Discussion
The anti-proliferative properties of the C.quadrangularis methanolic extract from aerial parts against MG63 cells were shown in this study using cytotoxicity assay. The assay detects the reduction of dimethylthiazole diphenyl tetrazolium bromide (MTT) salt to a coloured formazan product by mitochondrial enzyme succinate dehydrogenase, the intensity of the colour was measured using spectrophotometer, which measures the quantity of viable cells.16,17 The cell viability of MG63 cells decreased with increasing dose of the extract confirming its’ anti-cancerous property with IC50 value at around 100 µg/ml. Several naturally derived plant products with prospective anticancer properties against MG63 cells have already been reported by other authors and the reported IC50 values of other plant extracts compared to this study results suggest that C.quadrangularis methanolic extract exhibits considerable inhibition of MG63 cells.18,19
In a research work to study the anticancer activity of silver nanoparticles derived from seed extract of Capsicum sp, the IC50 values for two different extracts were 60.42 µg/ml and 64.99 µg/ml respectively.18 Plumbagin an active component of Plumbago zeylanica L. was studied for its anticancer activity against MG-63 osteosarcoma cell line. The IC50 concentration of plumbagin in MTT assay was observed to be 15.9 µg/ml.19 The incubation periods selected for these two studies were 48 hrs and 72 hrs respectively. In our study we have selected an incubation period of 24 hrs.
In an in-vitro study both chloroform as well as ethanol extract from the leaves of Cissus quadrangularis were compared, and studied for their anti-oxidant and anticancer activity. Ethanol extract was found to be better than chloroform extract for both the properties. The extract also showed potent anticancer activity against Ehrlich Adenocarcinoma cell lines which was demonstrated by MTT assay as well as tryptan blue method. The IC50 value of ethanol extract was found to be 150 µg/ml, and that of chloroform extract was 450 µg/ml in the MTT assay. The mechanism of cytotoxicity was postulated to be due to membrane damaging effect, and through activation of apoptotic pathways.20
The anticancer activity of alcoholic extract of the plant also have been demonstrated with various cell lines including HeLa (Cervical cancer), KB (Oral epidermoid carcinoma cell line), A431 (Skin epithelial carcinoma cell lines), MCF7 (Breast cancer cell line), HEp 2 (Human laryngeal carcinoma), HT29 (Colon carcinoma), and Vero cell line (Kidney epithelial cell). Inducing the production of reactive oxygen compounds in cancer cells, arresting the cell cycle at G1 phase by apoptosis activation were the possible proposed mechanisms of anticancer action.21-23
MG-63 osteosarcoma cell lines were utilized as a tool to assess the anticancer potential of molecules against skeletal malignancies. In a study conducted by Wang Jun 2017, the researchers demonstrated the anticancer activity of curcumin against MG-63 osteosarcoma cells. Considering the fact that these cells had p-53 mutation, which has led to uncontrolled proliferation, the anticancer effect of curcumin was considered to be by the action over the p-53 signalling pathway.24
The use of Cissus quadrangularis in the management of bone and joint disorders such as osteoporosis, osteoarthritis, and rheumatoid arthritis has been documented in native medicine. The antiosteoporotic potential of the ethanolic extract has been observed effectively in rat model of osteoporosis. The possible mechanism of this activity was attributed to its ability to enhance the differentiation of mesenchymal stem cells to osteoblasts, therefore enhancing bone formation. Wnt-β catenin pathway could be the target through which the plant extract exert its osteogenesis action.25 The same mechanism can have a contributing role in the anticancer action against osteosarcoma too.
Our study has shown that the methanolic extract of C.quadrangularis possess the potential to be a lead compound for further research for anticancer activity against osteosarcoma. We have not used an active control in MTT assay. Inclusion of an anticancer drug which is effective in treatment of osteosarcoma as a control, could have provided information about relative potency of our study compound and the possible mechanism of the same. Even though the phenolic constituent of the plant can be considered as the antiproliferative factor for the cytotoxicity observed, chromatographic characterization and quantification of the bioactive compounds present in the extract will broaden our knowledge and help us to optimize the compound for future research initiatives.
Conclusion
With the results of the present study, it may be inferred that methanolic extract of Cissus quadrangularis possess therapeutic potentiality against bone tumours. Further studies can be done on this plant extract to obtain more data for characterisation of responsible anticancer phytoconstituents, potential mode of action, as well as take the research forward for further exploration.
Acknowledgements
The author(s) received no specific funding to acknowledge for this research work.
Conflicts of Interest
There is no conflict of interest.
References
- Ferlay, H. R. Shin, F. Bray, D. Forman, C. Mathers, and D.M. Parkin, “Estimates of worldwide burden of cancer in 2008: GLOBOCAN2008,” International Journal of Cancer, vol. 127, no. 12, pp. 2893–2917, 2010.
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer. 2015 Mar 1;136(5):E359-86.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2018 Nov;68(6):394-424.
- Buolamwini J. K. Novel anticancer drug discovery. Current Opinion in Chemical Biology 1999;3(4):500–509.
- Shoeb, “Anticancer agents from medicinal plants,” Bangladesh Journal of Pharmacology, vol. 1, no. 2, pp. 35–41, 2006.
- Namiki, “Antioxidants/antimutagens in food,” Critical Reviews in Food Science and Nutrition, vol. 29, no. 4, pp. 273–300, 1990.
- Nagendra Prasad, H. Xie, J. Hao et al., “Antioxidant and anticancer activities of 8-hydroxypsoralen isolated from wampee [Clausena lansium (Lour.) Skeels] peel,” Food Chemistry, vol.118, no. 1, pp. 62–66, 2010.
- J. Balunas and A. D. Kinghorn, “Drug discovery from medicinal plants,” Life Sciences, vol. 78, no. 5, pp. 431–441, 2005.
- Dai and R. J. Mumper, “Plant phenolics: extraction, analysis and their antioxidant and anticancer properties,” Molecules, vol.15, no. 10, pp. 7313–7352, 2010.
- Chatterjee and S. Chandraprakash, The Treatise of Indian Medicinal Plants, vol. 3 of Publications and Information Directorate, CSIR,NewDelhi, India, 1997.
- Sen, M. K., and Dash, B. K. (2012). A review on phytochemical and pharmacological aspects of Cissus quadrangularis Int. J. Green Pharm. 6, 169–173.
- Ansarali S, Manikandan S, Alagulakshmanan GM. Review on Phytochemical and Pharmacological activities of the genus Cissus International Journal of Pharmaceutical Research. 2016. Volume 8, Issue 4, 1-7.
- Sha U. Cissus quadrangularis L.: Phytochemicals, traditional uses and pharmacological activities – a review. Int J Pharm Pharm Sci, Vol 3, Suppl 4, 41-44.
- Pautke C, Schieker M, Tischer T, Kolk A, Neth P, Mutschler W, Milz S. Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2 OS in comparison to human osteoblasts. Anticancer research. 2004 Nov 1;24(6):3743-8.
- Redfern J, Kinninmonth M, Burdass D, Verran J. Using soxhlet ethanol extraction to produce and test plant material (essential oils) for their antimicrobial properties. J Microbiol Biol Educ. 2014;15(1):45-6. Published 2014 May 1. doi:10.1128/jmbe.v15i1.656
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55-63.
- Abbas Momtazi-borojeni A, Behbahani M, Sadeghi-aliabadi H. Antiproliferative activity and apoptosis induction of crude extract and fractions of avicennia marina. Iranian journal of basic medical sciences 2013;16(11):1203.
- Singh N, Chatterjee A, Chakraborty K, Chatterjee S, Jayanthi A. Cytotoxic Effect on MG-63 Cell Line and Antimicrobial and Antioxidant Properties of Silver Nanoparticles Synthesized with Seed Extracts of Capsicum sp. Records of Natural Products. 2016;10(1):47.
- Yan CH, Li F, Ma YC. Plumbagin shows anticancer activity in human osteosarcoma (MG-63) cells via the inhibition of S-Phase checkpoints and down-regulation of c-myc. Int J Clin Exp Med. 2015 Aug 15;8(8):14432-9. eCollection 2015.
- Kumar A, Deepa B, Saravanan R, Hameed SAS. Reactive oxygen and nitrogen species scavenging and anticancer potential of Cissus quadrangularis against EAC cell line. Int J Pharm Pharm Sci. 2014 Sep 1;269–74.
- Rajamaheswari K, Visweswaran S, Muthukumar NJ, Murugesan M, Banumathi V. A Review on Anti- cancerous potential of Cissus quadrangularis. Int. J. Curr. Res. Chem. Pharm. Sci. 2017; 4(8): 1-3
- Kumar A, B D, Servanan R, Hameed SAS. Reactive oxygen and nitrogen species scavenging and anticancer potential of Cissus quadrangularis against EAC cell line. Int J Pharm Pharm Sci. 2014 Sep 1;269–74.
- Vijayalakshmi A, Kumar PR, Priyadarsini S, Meenaxshi C. In-vitro antioxidant and anticancer activity of flavonoid fraction from the aerial parts of Cissus quadrangularis against human breast carcinoma cell lines. Journal of Chemistry. 2013, Article ID 150675, 1-9.
- Jun W, Peng C, Wen J, Mingzhi G. Experimental study on curcumin inhibiting proliferation and invasion of human osteosarcoma cells. Biomed Res. 2017; 28 (10): 4396-4401.
- Joseph B, George J, Mohan J. Cissus quadrangularis in the treatment of osteoporosis. World Journal of Pharmaceutical Research. 2013. Volume 2, Issue 3, 596-605.








