Sulagna Dutta*1 and Pallav Sengupta2
and Pallav Sengupta2
1Department of Oral Biology and Biomedical Sciences, Faculty of Dentistry, MAHSA University, Malaysia.
2Department of Physiology, Faculty of Medicine, MAHSA University, Malaysia.
Corresponding Author E-mail: sulagna_dutta11@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1612
Abstract
Hamsters have unique physiological characteristics rendering them well-suited for biomedical research as experimental model. They match beneficial traits of both smaller rodents and larger mammals that make them suitable for laboratory use, such as availability, breeding ease, greater tissue proportions and the like. In experimental design, it is inevitable to select laboratory animals of accurate age that can mimic the target human age in a specific research. In this article, we have calculated that one human year equals 13.67 hamster days, considering their entire lifespan. This simplistic calculation may not find universal relevance in biomedical research, given the accelerated non-uniform life stages of hamsters when matched with human. To resolve this issue, this is the first ever article where we have provided a concise perception of hamster days in human years by correlating their age at every major life stage. This article will aid precision in biomedical research via selection of laboratory hamster of accurate age corresponding to human age, which is the most primary and essential criteria in animal based research.
Keywords
Age; Biomedical Research; Developmental Biology; Human Age; Laboratory Hamsters
Download this article as:| Copy the following to cite this article: Dutta S, Sengupta P. Age of Laboratory Hamster and Human: Drawing the Connexion. Biomed Pharmacol J 2019;12(1). |
| Copy the following to cite this URL: Dutta S, Sengupta P. Age of Laboratory Hamster and Human: Drawing the Connexion. Biomed Pharmacol J 2019;12(1). Available from: https://bit.ly/2FIP2v3 |
Introduction
Laboratory animals are the core requirements for biomedical research. Selection of laboratory animals should be as per specifications of the study and needs proper conception to relate the physiology and development of the laboratory animals with human. It is utmost crucial to establish the selection age criteria of the laboratory animal that will almost exactly mimic the age of human to which the research aims to actuate. Rodent models, namely mice and rats, are undoubtedly the most common laboratory animals owing to their small size, docility, adaptability, convenience in handling, low husbandry cost, fecundity, ease of genetic modulation and ready availability (Dutta and Sengupta, 2016; Sengupta, 2013). But other relatively large mammals find greater implications in some specific research that desire more physiological resemblance to human, which include rabbits, guineapigs and hamsters (Dutta and Sengupta, 2018; Iwatsuki-Horimoto et al., 2018; Padilla-Carlin et al., 2008).
Hamsters, rodents of the family Cricetidae (Clifford and Simmons, 2017), are extensively used for research on obesity, prostatic diseases, carcinogenesis (Kowshik et al., 2017), reproduction (Fujinoki and Takei, 2017; Lynch et al., 2017), infectious diseases (Marzi et al., 2018), diabetes (Hein et al., 2013), cardiovascular diseases (Glerup et al., 2017), dental caries (Lin et al., 2018), chronic bronchitis (Marshall et al., 2018), teratogenesis (Calado and dos Anjos Pires, 2018) etc. In 2010, the USA based research institutes that are registered under the Animal Welfare Act (AWA), reported the research use of 1,45,895 hamsters of different species and in 2016, 1,02,633 hamsters were used in research, which is about 13% of all the Animal Welfare Act-covered animals used in that year in the United States (NAVS, 2016). Due to implementation of more strict animal use regulations in recent years, the total animal usage for research has steadily declined by 8% since 1973 to 2016, while hamster use has shown an increase by 4% from 2015 to 2016 (NAVS, 2016). Hamsters are suited to be utilized for laboratory purpose as they are readily available, easy to breed and are also devoid of spontaneous diseases (Clifford and Simmons, 2017). Moreover, they find applicability in specific research owing to their greater physiological resemblance to human, larger volumes of blood and greater proportion of any tissues compared to smaller rodents; and less ethical and social complications as with the use of larger mammals (Wei et al., 2012; Yang et al., 2008).
As discussed, hamsters find immense application in various researches ranging from critical pathological conditions like carcinogenesis to most prevalent disorders like obesity. Hamster provides an analogous in vivo experimental model for these biomedical researches that ultimately aim to effectuate the result upon human. For this reason, the age of the experimental hamster should parallel the target human age of the specific research. This review aims to aid this precision in age selection of laboratory hamster corresponding to the human age. The non-uniformity between developmental stages of hamster and human makes it difficult to accurately correlate their age by merely comparing their individual entire life spans. Complying with our previous reports on age correlation of vital laboratory models with human age (Dutta and Sengupta, 2016, 2018; Sengupta, 2013), in this article we have considered every important life stage of both hamsters and humans to precisely present hamster days in human years.
Research use of hamsters
The reports from the United States Department of Agriculture display use of 102,633 hamsters in research in 2016, which is nearly 13% of all the Animal Welfare Act-covered animals used in that year (NAVS, 2016) (Figure 1). The most commonly used laboratory hamsters are the Syrian (golden) hamsters, followed by the English (black) and Chinese hamsters(Clifford and Simmons, 2017). Hamsters serve as ideal research models because they are largely available, easily and rapidly breed, develop quite fast, have short accelerated life stages and are readily susceptible to vast array of pathogens (Gao et al., 2014).
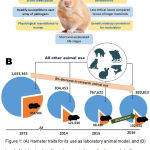 |
Figure 1: (A) Hamster traits for its use as laboratory animal model, and (B) trend of total animal and hamster uses in biomedical research (yellow and blue areas indicate respective usage of hamster and other animals in that fiscal year;
|
Data above the blue areas represent total animal use and data in the yellow areas represent total hamster use in research in that fiscal year).
Hamsters find wide variety of usage in research areas which include research on cancer, infectious disease and behavioural studies. They serve as genetic models for various human diseases such as epilepsy (Munoz et al., 2017), atrial thrombosis (Clifford and Simmons, 2017), and muscular dystrophy (I wata et al., 2018).
Hamster has some unique physiological characteristics that make it favourable to be used as laboratory model (Clifford and Simmons, 2017) (Table 1). Hamster has eversible cheek pouch which finds specific suitability in research on micro circulation and transplantation of adult, neonatal, and neoplastic tissues (Niwano et al., 2017). It is best suited as transplantation site owing to the direct accessibility of the cheek pouch for observation and is a specific privileged site exhibiting high immunological tolerance(Clifford and Simmons, 2017; Evangelista et al., 2017). Hamster teeth are also widely utilized in research associated with dental caries and various periodontal diseases (Niwano et al., 2017). The Syrian hamsters are particularly used in research concerned with the therapeutic and biochemical aspects of diseases and research in virology (Iwatsuki-Horimoto et al., 2018). Syrian hamsters are most commonly used in modulation of leptospirosis owing to their high susceptibility to leptospira (Evangelista et al., 2017). Chinese hamsters being usually genetically susceptible to diabetes mellitus, are mostly used in research related to diabetes (Clifford and Simmons, 2017), besides its usage in a variety of investigations in the realms of pathology and parasitology. The Chinese hamsters possess just 22 chromosomes, compared to 44 in the Syrian hamster and this attribute of the Chinese hamsters is used in cytological studies, involving tissue culture and evaluation of radiation effects and impacts of toxic substances (Rupp et al., 2018). Hamster is also an appropriate model for teratology research for its shorter gestation period (15-18 days) compared to other common laboratory rodents (Calado and dos Anjos Pires, 2018) (Table 2).
Table 1: General physiology and reproductive data of laboratory hamster (Syrian).
| Common physiological data | Reproductive data | ||
| Chromosome number (2n) | 44 (Syrian) 22 (European, Chinese) | Age at estrous | 6-8 weeks |
| Birth weight | 2-3 g | Duration of estrous | 4 days |
| Body temperature | 36.2-37.5oC | Breeding season | All year, may decrease in winter |
| Water consumption | 30 ml/day | Time of ovulation | Early estrous |
| Food consumption | 10-15 grams/day | Length of gestation | 15-18 days |
| Average litter size | 4-12 | Parturition interval | 65-85 days |
| Heart rate | 280-412 per minute | Average sperm volume | 0.5 ml |
| Respiratory rate | 33-127 per minute | Serum testosterone | 20-21 pmol/ml |
| Blood volume | 78 ml/kg body weight | Time of implantation | 5 or more days |
Table 2: Commonly used breeds of laboratory hamsters and their research applications.
| Mostly used breeds | Ideal mature weight (g) ( / / ) ) |
Average litter size | Duration of gestation (days) | Research applications |
| Syrian hamster (golden) | 85-140/95-120 | 4-12 | 15-18 | Cardiovascular diseases, muscular dystrophy, polycystic diseases, radiobiology research, hypothermia research, infectious diseases, tissue transplantation, viral and parasitology research |
| Chinese hamster | 50-75/55-70 | 4-5 | 20-21 | Diabetes mellitus, periodontal diseases, radiobiology research, tissue transplantation, genetic and teratology research, viral and parasitology research |
| European hamster | 150-400/200-550 | 4-12 | 18-21 | Respiratory diseases, smoke-inhalation studies, metabolic research, |
Age determination of laboratory hamsters
There are many methods in practice to determine ages of small mammals using various parameters which include the eye lens weight, body weight, pattern of tooth wear etc. The methods have respective limitations and can only provide approximation of the laboratory animal age (Dutta and Sengupta, 2016, 2018; Sengupta, 2013).
Eye Lens Weight
The variation in weight of the eye lens in different life stages of mammals provide a trustworthy parameter to predict the age of small laboratory mammals (Hardy et al., 1983). The eye lens weight increases obeying a proposed asymptotic curve through the entire life span of laboratory mammal (Lord, 1959). The limitations of this method lie in the fact that this is applicable only up to 3 to 4 months of the animal’s life (Friend, 1967).
Musculoskeletal Parameters: Epiphyseal Closure
There are proposed formulae correlating bone length and age and can serve as a reliable parameter if the measurements are precise. In the experimental animals, prediction of age is mediated mainly via analysis of the upper and lower limb bones and those of the hip joints. In the pre-pubertal phase of laboratory animal, there is metopic suture closure and ossific centers emerge which serve as their age indicators. Growth of the epiphyseal plates as well as their closure indicates the onset of sexual life in most of the mammals (Kilborn et al., 2002). In humans, epiphyseal closure in the upper body portions (namely: shoulder joint, humerus, ulna, radius wrist, metacarpals and phalanges) are observed at 14-18 years of age, while the lower portions (tibia and femur) close during the age of 18-25 years. Early adulthood is characterised by bone remodelling and maintenance, while late adulthood by bone wears and tears. Epiphyseal evaluation involves detailed analysis of skeletal remains and radiological interventions of the fleshed material (Kohn et al., 1997).
Body Weight Assessment and Physical Attributes
Different age classes of the small laboratory mammals, such as mice, rats or hamsters, can be predicted by plotting the frequency distribution of their body weights segregating into different cohorts and thereby determining the body weight distribution by statistical models (Chou et al., 1998). The approximate age of hamster pups can also be predicted via physical traits alterations during the primary two weeks of their life.
Tooth Wear Pattern
A laboratory rodent suffers constant molar tooth attrition, and the degree of tooth wear is considered proportional to the rodent’s age(Chou et al., 1998). With grinding and biting actions, the hamsters undergo continuous wearing of teeth. The pattern of tooth wear has been studied extensively and compared among various animals to standardize it as parameter to predict rodent age (Klingsberg and Butcher, 1960). The grinding surface height of the posterior molar (M3) in the hamsters along with the molar wear has been documented as better age predictor in laboratory hamsters compared to other common parameters such as body weight and body length(Haoquan et al., 1987).
Relation Between Hamster Age and Human Age
Laboratory hamsters are used in biomedical research as experimental models representing humans and thereby their age must precisely correspond to the age of human which the research intends to serve. In the following section, we present human age with the age of laboratory hamsters at different life stages (Figure 2).
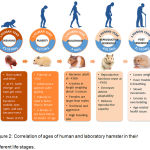 |
Figure 2: Correlation of ages of human and laboratory hamster in their different life stages.
|
Relation Between the Lifespan of Laboratory Hamster and Human
Hamsters have active short accelerated life-stages which cannot be coincided with that of humans. Due to the non-uniformity of the hamster developmental stages with humans, their age correlation may not be of universal relevance if their entire individual life spans are taken into account.
The average lifespan of a laboratory hamster is around 3 years (Bartlett, 2015), where as human may live on an average for 80 years (Dutta and Sen gupta, 2016, 2018; Sen gupta, 2013).
Therefore, the age correlation between hamster and human on the basis of their entire life span can be calculated as follows:
(80 × 365) ÷ (3 × 365) = 26.7 human days = 1 hamster day; and 365 ÷ 26.7 = 13.67 hamster days = 1 human year.
Thus, one human year is almost equivalent to 13.67 hamster days on the basis of their entire life span.
Weaning Period of Hamster and Human
Mammals nurse and feed the newly born young ones till they are able to withdraw from mother’s milk and can survive independently. ‘Weaning is the transition of the human infant from breast-feeding or bottle nursing and commencement of nourishment with other food’ (Fagundes and Taha, 2004). The hamster litters or pups, grow very rapidly. While born, they are naked and blind. At around P7, there is emergence of teeth from their gums, and the pups also develop hair with visible distinct coloration. Between P11 and P14, the pups become voluntarily mobile, their eyes begin to open up and ears become erect. These are soon followed by full-furred appearance. At this point, the hamster pups besides being sill fed by their mother, also may consume few other soft foods (Clifford and Simmons, 2017), (Bartlett, 2015). In the third week post birth, hamster pups resemble miniature adults. Since female hamsters usually conceive at their post-partum estrus, the hamster pups should be weaned from their mother at P18 or P19 (Wynne-Edwards, 1987). While in the wild, hamster pups reportedly wean at around P-23, leaving their home nest and thereby forage on their own, and establish nest sites, and defend their territory (Schoenfeld and Leonard, 1985).
Weaning in hamsters usually vary from P18 to P23, while weaned mostly on 21st day (P21), after birth. While, the average weaning age for humans is about 6 months (180 days) (Dutta and Sengupta, 2016, 2018; Sengupta, 2013).
Thus, 180 ÷ 21 = 8.87 human days = 1 hamster day and 365 ÷ 8.57 = 42.59 hamster days = 1 human year.
Therefore, in this developmental phase, one human year equals 42.59 hamster days.
Hamster and Human Age to Attain Puberty
Puberty is characterised by maturation of hypothalamo-pituitary-gonadal axis, alterations in gonadotropin and sex steroids levels. Since hamsters live only for few years, adolescence appears quite quickly. Hamsters usually reach their sexual maturity at about 6th weeks after birth, and puberty in male appears faster than in females. A female is not generally bred before she is 10 weeks old owing to higher incidence of stillbirths.The average age at which hamsters attain puberty is about P42 (Ferris and Brewer, 1996). Humans attain their pubertal age at about 11.5 years (11.5 × 365 = 4198 days) (Dutta and Sengupta, 2016, 2018; Sengupta, 2013).
Thus, in the prepubertal phase, 4198 ÷ 42 = 99.95 human days = 1 hamster day, and 365 ÷ 99.95 = 3.65 hamster days = 1 human year.
Thus, in this phase, one human year is equivalent to 3.65 hamster days.
Adulthood in Hamsters and Human
Adulthood refers to the age of sexual maturity associated with maturity in physiological, reproductive and psychological aspects. Hamsters attain sexual maturity approximately at 6 months of age or P180 (Calvo et al., 1997). They grow approximately to 6 inches in length weighing about 3 to 5 ounces. Female hamsters are most often larger than the males.
The early adulthood in humans is considered to begin at around 20 years of age (365 × 20 = 7300 days) (Kilborn et al., 2002).
Therefore, from these data, it can be calculated that
7300 ÷ 180 = 40.55 human days = 1 hamster day, which indicates that 365 ÷ 40.55 = 9 hamster days = 1 human year.
Thus, during the adult phase, nine hamster days are equivalent to one human year.
Reproductive Senescence in Hamsters and Humans
In hamsters, reproductive functions cease in late middle-age at around 1 years and more specifically when they are 15 months old, P450 (Blaha, 1964). In female hamsters it is reported that decreasing uterine adaptability corresponds to their reduced reproductive capacity during senescence (Blaha, 1964). In humans, menopause is considered to be the reliable marker of reproductive senescence, which refers to the termination of fertility cycle in women which is 51 years (51 × 365 = 18,615 days), according to the American Medical Association (Durbin et al., 1966).
Thus, 18,615 ÷ 450 = 41.37 human days = 1 hamster day, and 365 ÷ 41.37 = 8.82 hamster days = 1 human year.
Thus, during reproductive senescence, 8.82 hamster days are equivalent to one human year.
Post-Senescence Phase in Hamsters and Humans
Hamsters live maximum of about 21 months (P630) after the occurrence of reproductive senescence in them. The old age in hamsters render them thin, with less body hair, and most commonly troubled breathing, much slower movements and faded lustres in the eyes. The post-senescent survival in the human may be 10,585 days in average (Dutta and Sengupta, 2016, 2018; Sengupta, 2013).
Thus, 10585 ÷ 630 = 16.80 human days = 1 hamster day, 365 ÷ 29 = 21.73 hamster days = 1 human year.
Thus, in the senescence phase, 21.73 hamster days are equivalent to one human year.
Conclusion
Hamsters are one of the most important laboratory animals and indispensable for some specific biomedical research which exploit their unique physiological traits to effectuate the research outcomes on human. Selection of laboratory hamster thereby needs proper knowledge of its characteristics in successive life stages. Biomedical research using animal models such as hamsters targets particular age in human to apply its results. Thus, it is very essential to have a clear perception of hamster days in human years. Considering the disparity of a minute accelerated hamster life with elaborate human life, this article provides a comprehensive age correlation of laboratory hamster with that of human at every vital life stage to ensure more accuracy in age selection of laboratory model.
Acknowledgements
The author(s) received no specific funding for this work.
Conflict of Interest
There is no conflict of interest.
Funding Source
As it is a review article, no financial support has been taken from any authority.
Ethical Approval
Not applicable.
References
- Bartlett P. P. The hamster handbook, in: Bartlett P. (Ed.), Barron’s Educational Series 2nd ed. Barron’s Educational Series. 2015.
- Blaha G. C. Reproductive senescence in the female golden hamster. The Anatomical Record. 1964;150:405-411.
CrossRef - Calado A. M., dos Pires M. A. An Overview of Teratology, in: Felix, L. (Ed.), Teratogenicity Testing 1st ed. Springer. 2018;XV:614.
CrossRef - Calvo A., Martinez E., Pastor L. M., Vazquez J. M., Roca J. Classification and quantification of abnormal sperm along the epididymal tract. Comparison between adult and aged hamsters. Reproduction, nutrition, development. 1997;37:661-673.
CrossRef - Chou C., Lee P., Lu K., Yu H. A Population Study of House Mice (Mus musculus castaneus) Inhabiting Rice Granaries in Taiwan. Zoological Studies. 1998;37:201-212.
- Clifford C. B., Simmons J. H. The Laboratory Hamster, in: Kurtz D. M., Travlos G. S. (Eds.), The Clinical Chemistry of Laboratory Animals, 3rd ed. CRC Press, Taylor and Francis, Philadelphia. 2017.
- Durbin P. W., Williams M. H., Jeung N., Arnold J. S. Development of spontaneous mammary tumors over the life-span of the female Charles River (Sprague-Dawley) rat: the influence of ovariectomy, thyroidectomy and adrenalectomy-ovariectomy. Cancer research. 1966;26:400-411.
- Dutta S., Men P. S and mice: Relating their ages. Life Sci. 2016;152:244-248.
CrossRef - Dutta S., Sengupta P. Rabbits and men: relating their ages. Journal of basic and clinical physiology and pharmacology. 2018.
CrossRef - Evangelista K. V., Lourdault K., Matsunaga J., Haake D. A. Immunoprotective properties of recombinant Lig A and LigB in a hamster model of acute leptospirosis. PloS one. 2017;12:0180004.
CrossRef - Fagundes D. J., Taha M. O. Animal disease model: choices criteria and current animals specimens Acta Cir. Bras. 2004;19:59-65.
CrossRef - Ferris C. F., Brewer J. Adolescent stress alters ethanol ingestion and agonistic behavior in the male golden hamster. Annals of the New York Academy of Sciences. 1996;794:348-351.
CrossRef - Friend M. A review of research concerning eyelens weight as a criteria of age in animals. New York Fish Game Journal. 1967;14:152-165.
- Fujinoki M., Takei G. L. gamma-Aminobutyric acid suppresses enhancement of hamster sperm hyperactivation by 5-hydroxytryptamine. The Journal of reproduction and development. 2017;63:67-74.
CrossRef - Gao M., Zhang B., Liu J., Guo X., Li H., Wang T., Zhang Z., Liao J., Cong N., Wang Y., Yu L., Zhao D., Liu G. Generation of transgenic golden Syrian hamsters. Cell research. 2014;24:380.
CrossRef - Glerup S., Schulz R., Laufs U., Schluter K. D. Physiological and therapeutic regulation of PCSK9 activity in cardiovascular disease. Basic research in cardiology. 2017;112:32.
CrossRef - Haoquan L., Yuchun L., Xuedong Z. Age determination, age structure and population dynamics of striped hamster (Cricetulus barabensis). Acta Theriologica Sinica . 1987;1:004.
- Hardy A. R. R. J., Q., L.W., H. Estimation of age in the Norway rat (Rattusnorvegicus) from the weight of the eyelens. Journal of Applied Ecology. 1983;20:97-102.
CrossRef - Hein G. J., Baker C., Hsieh J., Farr S., Adeli K. GLP-1 and GLP-2 as yin and yang of intestinal lipoprotein production: evidence for predominance of GLP-2-stimulated postprandial lipemia in normal and insulin-resistant states. Diabetes. 2013;62:373-381.
CrossRef - Iwata Y., Katayama Y., Okuno Y., Wakabayashi S. Novel inhibitor candidates of TRPV2 prevent damage of dystrophic myocytes and ameliorate against dilated cardiomyopathy in a hamster model. Oncotarget. 2018;9:14042-14057.
CrossRef - Iwatsuki-Horimoto K., Nakajima N., Ichiko Y., Sakai-Tagawa Y., Noda T., Hasegawa H., Kawaoka Y. Syrian Hamster as an Animal Model for the Study of Human Influenza Virus Infection. Journal of virology. 2018;92.
- Kilborn S. H., Trudel G., Uhthoff H. Review of growth plate closure compared with age at sexual maturity and lifespan in laboratory animals. Contemporary topics in laboratory animal science. 2002;41:21-26.
- Klingsberg J., Butcher E. O. Comparative histology of age changes in oral tissues of rat, hamster, and monkey. Journal of Dental Research. 1960;39:158-169.
CrossRef - Kohn L. A., Olson P., Cheverud J. M. Age of epiphyseal closure in tamarins and marmosets. American journal of primatology. 1997;41:129-139.
CrossRef - Kowshik J., Mishra R., Sophia J., Rautray S., Anbarasu K., Reddy G. D., Dixit M., Mahalingam S., Nagini S. Nimbolide upregulates RECK by targeting miR-21 and HIF-1α in cell lines and in a hamster oral carcinogenesis model. Scientific Reports. 2017;7:2045.
CrossRef - Lin T. H., Lin C. H., Pan T. M. The implication of probiotics in the prevention of dental caries. Applied microbiology and biotechnology. 2018;102:577-586.
CrossRef - Lord D. R. The lens as an indicator of age in cotton-tail rabbits. Journal of Wildlife Management. 1959;23:358-360.
CrossRef - Lynch E. W., Coyle C. S., Stevenson T. J. Photoperiodic and ovarian steroid regulation of histone deacetylase 1, 2, and 3 in siberian hamster (Phodopus sungorus) reproductive tissues. General and Comparative Endocrinology. 2017;246:194-199.
CrossRef - Marshall B. C., Rao N. V., Niewoehner D. E., Hoidal J. R. Cadmium Chloride-Induced Emphysema in Lathyrogenic Hamsters, in: Cantor J. O. (Ed.), CRC Handbook of Animal Models of Pulmonary Disease. CRC Press, Taylor and Francis, Philadelphia. 2018;15-28.
- Marzi A., Haddock E., Kajihara M., Feldmann H., Takada A. Monoclonal Antibody Cocktail Protects Hamsters From Lethal Marburg Virus Infection. The Journal of infectious diseases. 2018.
CrossRef - Munoz L. J., Carballosa-Gautam M. M., Yanowsky K., Garcia-Atares N., Lopez D. E The genetic audiogenic seizure hamster from Salamanca: The GASH:Sal. Epilepsy & behavior : E&B . 2017;71:181-192.
CrossRef - NAVS. Hamsters in research. National Anti-vivisection Society, Chicago, United States. 2016.
- Niwano Y., Konno K., Matayoshi T., Nakamura K., Kanno T., Sasaki K. Oral mucosal irritation study in hamster to evaluate a therapeutic apparatus using hydrogen peroxide photolysis for periodontitis treatment. Regulatory toxicology and pharmacology : RTP. 2017;90:206-213.
CrossRef - Padilla-Carlin D. J., McMurray D. N., Hickey A. J. The guinea pig as a model of infectious diseases. Comparative medicine. 2008;58:324-340.
- Rupp O., MacDonald M. L., Li S., Dhiman H., Polson S., Griep S., Heffner K., Hernandez I., Brinkrolf K., Jadhav V., Samoudi M., Hao H., Kingham B., Goesmann A., Betenbaugh M. J., Lewis N. E., Borth N., Lee K. H. A reference genome of the Chinese hamster based on a hybrid assembly strategy. Biotechnology and bioengineering. 2018;115:2087-2100.
CrossRef - Schoenfeld T., Leonard C. Behavioral Development in the Syrian Golden Hamster, in: Siegel H. (Ed.) The Hamster, 1st ed. Springer US, United States.1985;458.
CrossRef - Sengupta P. The Laboratory Rat: Relating Its Age With Human’s. International journal of preventive medicine. 2013;4:624-630.
- Wei J., Ouyang H., Wang Y., Pang D., Cong N.X., Wang T., Leng B., Li D., Li X., Wu R., Ding Y., Gao F., Deng Y., Liu B., Li Z., Lai L.,Feng H., Liu G., Deng X. Characterization of a hypertriglyceridemic transgenic miniature pig model expressing human apolipoprotein CIII. The FEBS journal. 2012;279:91-99.
CrossRef - Wynne-Edwards K. E. Evidence for obligate monogamy in the Djungarian hamster, Phodopus campbelli: pup survival under different parenting conditions. Behavioral Ecology and Sociobiology. 1987;20:427-437.
CrossRef - Yang S. H., Cheng P. H., Banta H., Piotrowska-Nitsche K., Yang J. J., Cheng E. C., Snyder B., Larkin K., Liu J., Orkin J., Fang Z. H., Smith Y., Bachevalier J., Zola S. M., Li S. H., Li X. J., Chan A.W. Towards a transgenic model of Huntington’s disease in a non-human primate. Nature 2008;453:921-924.
CrossRef








