D. I. Pozdnyakov*1 , Z. D. Khadzieva2
, Z. D. Khadzieva2 , A. E. Pozdnyakova2
, A. E. Pozdnyakova2  and N. S. Zagorskaya2
and N. S. Zagorskaya2
1Department of pharmacology with course of clinical pharmacology, Pyatigorsk Medical and Pharmaceutical Institute, a branch Volgograd State Medical University, Pyatigorsk, (357532, Pyatigorsk, av. Kalinina 11), Russia.
2Department of pharmaceutical technology with course biotechnology, Pyatigorsk Medical and Pharmaceutical Institute, a branch Volgograd State Medical University, Pyatigorsk, (357532, Pyatigorsk, av. Kalinina 11), Russia.
Corresponding Author E-mail: pozdniackow.dmitry@yandex.ru
DOI : https://dx.doi.org/10.13005/bpj/1660
Abstract
To assess the severity of the anti-allergic effect of the combined nasal spray in the conditions of experimental allergic rhinitis. The experiment was performed on Balb / c male mice, which reproduced ovalbumin-induced allergic rhinitis. The test-spray composition of fexofenadine hydrochloride + ammonium glycyrrhizinate in doses of 2.5 μg; 5 μg and 7.5 μg / nostril and compartion drugs: beclomethasone («Nasobec», IVAX Pharmaceuticals) and levocabastine («Tyzine® Allergy», Johnson & Johnson) in doses of 3.5 μg / nostril and 5 μg / nostril respectively, were administered intranasally after 14-day immunization of animals. On the 17th day of the experiment, the severity of nasal symptoms (sneezing and nasal grooming), the change in the concentration of histamine, IFN-γ, IL-6, IgE, and TNF-α and markers of oxidative stress (superoxide dismutase activity and concentration of malonic dialdehyde) were determined. The use of levocabastine and beclomethasone contributed to the reduction of allergic symptoms, with the most pronounced pharmacological effect observed with the administration of beclomethasone. The administration of the 5 μg of test-spray reduced nasal symptoms in mice and also contributed to a decrease in the concentration of histamine, IFN-γ, IL-6, IgE, and TNF-α, as well as the restoration of pro / antioxidant balance. At the same time, the test aerodisperse system at a dose of 5 μg was comparable to beclomethasone and exceeded levocabastine in terms of pharmacological action. The high effectiveness of the test-spray, comparable to itranasal glucocorticoids, makes this compound a promising drug corrector of allergic rhinitis.
Keywords
Allergic Rhinitis; Antiallergic Drugs; Fexofenadine
Download this article as:| Copy the following to cite this article: Pozdnyakov D. I, Khadzieva Z. D, Pozdnyakova A. E, Zagorskaya N. S. Antiallergical Effect of New Combined Nazal Aerodisperse System in the Conditions of Experimental Allergic Rhinitis. Biomed Pharmacol J 2019;12(1). |
| Copy the following to cite this URL: Pozdnyakov D. I, Khadzieva Z. D, Pozdnyakova A. E, Zagorskaya N. S. Antiallergical Effect of New Combined Nazal Aerodisperse System in the Conditions of Experimental Allergic Rhinitis. Biomed Pharmacol J 2019;12(1). Available from: https://bit.ly/2OkFBob |
Introduction
Allergic rhinitis (AR) is a disease of the respiratory system, which is based on the pathogenesis of inflammation of the mucous membrane of the nasal cavity of allergic genesis.1 According to the World Health Organization, the AR is the main early form of progressive hypertrophic rhinitis, which accounts for 10–20% of cases of primary lesions of the nasal cavity.2 Up to 400 million cases of AR are recorded annually, and this pathology is the leading allergic nosology in both adults and children.3 Typical symptoms of AR are usually limited to nasal symptoms and include: rhinorrhea, sneezing, itching of the paranasal sinuses, swelling of the mucous membrane and nasal congestion.4 Despite the mild course of the AR, this disease causes a significant deterioration in the quality of life of the population, which increases the socioeconomic burden on the health systems of developed and developing countries. Thus, a number of studies have proved a direct relationship between the presence of AR and the subsequent development of allergic neurodermatitis, or bronchial asthma, and it is quite important that AR is the leading adverse prognostic sign of allergic hyperreactivity of the bronchopulmonary system.5 In addition to complications of the respiratory system, AR contributes to the development of a number of non-respiratory pathological changes. At the same time, obstruction of the nasal passages, the small bronchi and the associated pulmonary hypoventilation contribute to the development of sleep apnea and disturbed sleep patterns, worsening the general condition of the body, reducing mental and physical performance, depression.6 In addition, the neuropsychiatric symptoms accompanying AR are not limited to the syndromes listed above. It has been established that in the conditions of AR, an activation of the hypothalamic-pituitary-adrenal system with hyperproduction of glucocorticosteroids causing an increase in the body’s emotional reactivity, an increase in stressogenicity and anxiety level that reaches a pathological level.7 At the same time, neuropsychiatric disorders in people suffering from respiratory allergies are noted at the age of 20-40, i.e. in persons of the most working age.8 In addition to mental disorders, AR is directly related to the exacerbation of somatic pathologies. So it is described that in patients with AR, there is an increased activity of the parasympathetic nervous system, which in turn can lead to exacerbation of gastroesophageal reflux, peptic gastric ulcers, arrhythmias and obesity.9 In many ways, the complications of AR are
wood was used as a litter material. The water supply was carried out through standard 250 ml containers with free access. The animals received food and drinking water ad libitum. The underlay and watering tanks changed 1 time in 3 days. Placement, maintenance and subsequent manipulations with animals complied with the requirements of the European Convention for the Protection of Vertebrate Animals used for experiments and other scientific purposes (Strasbourg, 1986). The study was linked with the pathogenesis of this disease and the cyto – and chemokines formed during the pathobiochemical reactions.
The pathogenesis of AR is a classic variant of the reagin form of an allergic reaction. In response to the action of an allergen in the nasal mucosa, the chemotaxis of immunologically reactive cells is enhanced: CD-4 positive cells, eosinophils, mast cells, macrophages, T and B-lymphocytes, which infiltrate the nasal lining of the mucosa. At the same time, an extensive pool of cytokines (IL-3; IL-4; IL-6, TNF-α), as well as IFN-γ, is released. The resulting proallergic agents stimulate antibody production, and as a result of produced IgE through interaction with mast cells via high affinity FceR1 receptors enhances the release of mediators – histamine, leukotrienes, which in turn cause the typical allergic symptoms: swelling of the nasal mucosa due to arterial vasodilation and increase in vascular permeability, rhinorrhea, hyperemia of the paranasal sinuses, obstruction of the nasal passages.10 In addition, IFN-γ is an initiating pro-apoptotic signal that mediates desquamative processes in the epithelium of the nasal passages, which aggravates the obstruction of the respiratory tract and rhinorrhea.11
Strategies for the treatment of AR are primarily based on the features of the pathogenesis of this nosology and are mainly symptomatic. First-line drugs are topical glucocorticoids (beclomethasone, mometasone, budesonide, fluticasone) for intranasal use, showing a high therapeutic efficacy, especially in patients with burdened or resistant AR.12 However, glucocorticoids versus efficacy do not have an optimal toxic profile, and their use is associated with the appearance of a significant number of undesirable drug reactions, or the toxic effect of drugs may be unpredictable.13 In addition to glucocorticoids, oral antihistamines (fexofenadine, desloratadine), which do not have a sedative and M-anticholinergic effect, are used in the treatment of AR, but oral administration of these drugs also does not preclude the occurrence of adverse reactions.14 Thus, the development of new nasal anti-allergic drugs can be a new vector of AR pharmacotherapy, combining a high degree of efficacy and safety of use.5
Materials and Methods
Experimental Animals and Their Maintenance
The work was performed on 70 male mice of the Balb / c line weighing 20-22 grams, obtained from the vivarium PMPhI – a branch of FSBEI HE VolgGMU. At the time of the experiment, the animals were kept in controlled conditions: ambient air temperature of 20-220 C, relative humidity of 60-65%, with a natural change of the light-dark regime. The placement of animals in macrolon cells (type T / 4B, dimensions 580x375x200 mm) in 10 individuals eliminated crowding and stressogenicity. A granulated wood fraction of non-coniferous approved by the local ethics committee (protocol No. 24 of 09/13/2018).
Test-Objects
We studied the antiallergic effect of the new combined aerodisperse system for intranasal administration (nasal spray), based on the main active ingredients: fexofenadine hydrochloride and ammonium glycyrrhizinate. Levocabastine («Tyzine® Allergy», Johnson & Johnson) and beclomethasone («Nasobec», IVAX Pharmaceuticals) were used as reference drugs. The injection of the test-spray and the comparison preparations was administered intranasally, using the appropriate atraumatic nasal applicator for mice. The aerodisable system under study was administered in the form of a 0.05% solution in doses: 2.5 μg / nostril; 5 µg / nostril and 7.5 µg / nostril. Levocabastine was used at a dose of 5 µg / nostril,15 beclomethasone was administered at a dose of 3.5 µg / nostril.16
Model of Allergic Rhinitis. Study design.
Allergic rhinitis in animals (n = 60) was reproduced by the ovalbumin-induced method. Mice were immunized for 14 days with daily intraperitoneal injection of 1% ovalbumin solution (OVA, Panreac, Spain) with aluminum hydroxide (alum) in a 1: 1 ratio in an amount of 0.2 ml. On the 15th, 16th and 17th day of the experiment, animals were provoked by acute allergic rhinitis, by intranasal instillation of an OVA solution for immunization in a volume of 25 μl in each nostril.17 Previously (30 minutes before the provocation of rhinitis), intranasal administration of the test-spray and comparison preparations was carried out, for which the following experimental groups were formed (n = 10 each group): intact animals (mouse) (IM), in whom immunization was not carried out; a group of negative control mice (NC) – after immunization, this group received a distilled water in a volume of 10 μl in each nostril; groups of animals No. 3-5 to which the test-spray was administered in various dose (10 μl / nostril); groups of mice No. 6 and No. 7, which received reference preparations (levocabastine and beclomethasone, respectively, 10 μl / nostril). After the last instillation (on the 17th day of the experiment), nasal symptoms of AR were recorded in the mice, and biological material was taken for ELISA studies and for determining concentrations of oxidative stress markers (malonil dialdehyde (MDA) concentrations and superoxide dismutase (SOD) activity) Assessment of nasal symptoms of AR
Assessment of nasal symptoms of AR was performed on the 17th day of the experiment after the 3rd intranasal administration of the OVA solution. The animals were placed in individual cages and after nasal administration of the OVA solution, the number of sneezing and nasal grooming was recorded for 10 minutes.
Biomaterial
As a biomaterial in the work used the serum of mice. Blood sampling was performed in anesthetized animals (chloral hydrate, 350 mg / kg intraperitoneally) from the abdominal aorta into vacutainer type tubes, with citrate filling. For the ELISA study, sample preparation was performed by centrifuging whole blood in the mode of 20 min., 1000g. The analysis was performed immediately. Blood serum for the determination of SOD activity and the content of MDA were obtained by centrifuging the blood at 3000 rpm, 10 min. The volume of aliquots for the ELISA study was 100 μl, to assess changes in the activity of SOD and the concentration of MDA – 15 μl.
ELISA Study
The enzyme immunoassay was carried out using species-specific reagent kits manufactured by Cloud clone (Houston, USA) in a solid-phase (ELISA) embodiment, according to the analysis procedure prescribed in the manufacturer’s instructions. At the same time, the concentrations of histamine, immunoglobulin E (IgE), γ-interferon (IFN-γ), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) served as determinants. The results were read using an Infinite F50 microplate ELISA reader system (Tecan, Austria).
Determination of Superoxide Dismutase Activity
The evaluation of SOD activity was carried out by xanthine-xanthioxidase method. The principle of the method is based on the ability of SOD to catalyze the dismutation reaction of a superoxide radical generated as a result of the xanthine oxidation reaction, and to prevent the transition of 2- (4-iodophenyl) -3- (4-nitrophenol) -5-phenyltetrazolium chloride (I.N.T) red- painted formazan. The incubation medium contained: xanthine – 0.05 mmol / l; I.N.T. – 0.025 mmol / l; CAPS-EDTA buffer solution with pH 10.2; xanthine oxidase 80 U / L The optical density of the mixture was recorded at 505 nm. The enzyme activity was expressed in U / L.18
Determination of Malonil dialdehyde Concentration
The content of MDA in the serum of mice was determined in a condensation reaction with 2-thiobarbituric acid, during which a pink-colored trimethine complex is formed, which has an absorption maximum at 532 nm. Pre-protein in the sample precipitated by the addition of a 17% solution of trichloroacetic acid in the ratio 2: 1. Next, the resulting mixture was centrifuged in the mode 400g for 10 minutes, after which a 0.8% solution of 2-thiobarbituric acid (2: 1) was added to the supernatant and placed for 10 minutes in a boiling water bath. Upon reaching the specified time interval, the samples were transferred to micro cuvettes for analysis and the optical density of the mixture was recorded at 532 nm. To calculate the concentration of MDA, a molar extinction coefficient of 1.56 * 105 lmol-1cm-1 was used. Results are expressed in nmol MDA / L.19
Statistical Analysis
Statistical processing of the obtained results was performed using the STATISTICA 6.0 software package. Data were expressed as M ± SEM (mean ± standard error of the mean). Comparison of means was performed using the ANOVA method with the Newman-Keuls post-test. Differences were considered statistically significant at p <0.05.
Results
Evaluation of Nasal Symptoms
The results of the assessment of nasal symptoms of AR in mice are presented in Fig.1. In animals of the NC group, an increase in the number of sneezing and nasal grooming was observed relative to the IM group by 25.3 times (p<0.05) and 11.8 times (p<0.05), respectively. Introduction of reference drugs levocabastine and beclomethasone contributed to the reduction of nasal symptoms of AR in mice, which was reflected in a decrease in the number of sneezing and nasal grooming in relation to the NC group of animals in mice treated with levocabastine by 35.7% (p<0.05) and 51,6% (p<0.05), respectively, and in animals administered with beclomethasone — by 153.3% (p<0.05) and 104.3% (p<0.05), respectively. Under the conditions of nasal instillation of the test-spray at a dose of 2.5 μg, a decrease in the number of sneezing and nasal grooming was observed in comparison with the NC group of animals by 80.9% (p <0.05) and 56.7% (p <0.05) respectively. At the same time, the introduction of the test-spray at a dose of 5 μg caused a decrease in the nasal symptoms of AR (sneezing and paranasal grooming) relative to the NC group of mice by 230.4% (p<0.05) and 161.1% (p<0.05), respectively . At the same time, the indices of the group of animals that received the test composition at a dose of 5 μg did not statistically significantly differ from the analogous parameters of the group of mice that were nasally administered beclomethasone and were 143.5% (p<0.05) (sneezing) and 66.7% (p <0.05) (acts of grooming) is smaller compared with the group of animals that were adjusted for AR by levocabastine. The use of the spray in a dose of 7.5 μg contributed to a decrease in the number of sneezing and nasal grooming in relation to the NC group of mice by 100% (p <0.05) and 74.1% (p <0.05), respectively.
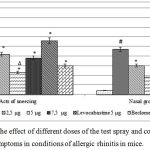 |
Figure 1: Assessment of the effect of different doses of the test spray and comparison drugs on the manifestation of nasal symptoms in conditions of allergic rhinitis in mice.
|
Note: IM – a group of intact animals; NC – a group of animals of negative control; # – statistically significant relative to the IM group of mice (p<0.05, Newman-Keuls test); * – statistically significant relative to the NC group of animals (p<0.05, Newman-Keuls test); ∆ – statistically significant relative to the group of mice treated with levocabastine (p<0.05, Newman-Keuls test)
Evaluation of Changes in SOD Activity and MDA Concentration
The results of the evaluation of the change in SOD activity and the concentration of MDA on the background of the correction of AR by the studied composition in various dosing options, as well as by the comparison drugs are presented in table 1.
In animals of the NC group under AR conditions, there was an increase in the concentration of MDA in the serum compared to the IM group of mice by 6.5 times (p<0.05), as well as a decrease in SOD activity by 3.9 times (p<0.05). The use of levocabastine and beclomethasone concentration of MDA decrease to relative to the NC group of animals by 85% (p<0.05) and 302.7% (p<0.05), respectively, but at the same time, the introduction of reference drugs did not have a significant effect on SOD activity. Against the background of application of the test-spray at a dose of 2.5 μg, a decrease in the concentration of MDA and an increase in SOD activity in the serum of mice was observed in comparison with the NC group of animals by 63.6% (p<0.05) and 98.6% (p<0,05) respectively, at the same time, the application of the test-spray at a dose of 5 μg contributed to a decrease of a MDA content in relation to the NC group of mice by 199% (p<0.05) and an increase in SOD activity by 106% (p <0.05). Also with the introduction of the tested spray in a dose of 5 μg, the MDA content was lower, and the activity of SOD, respectively, was higher compared to animals treated with levocabastine by 61.4% (p<0.05) and 81.6% (p<0.05), in addition, the activity of SOD in the application of the studied composition at a dose of 5 μg exceeded that of mice receiving beclomethasone by 64.1% (p <0.05). In terms of the introduction of the investigated composition at a dose of 7.5 μg, there was a decrease in the concentration of MDA relative to the NC group of animals by 85% (p <0.05), while the SOD activity increased by 89% (p<0.05).
Table 1: Changes in the concentration of MDA and SOD activity in the conditions of correction of allergic rhinitis with the test-spray in different dosage options and comparison drugs.
| Group | IM | NC | 2,5 μg | 5 μg | 7,5 μg | Levocabastine 5 μg | Beclomethasone 3,5 μg |
| MDA, nmol/L | 0,91±
0,036 |
5,89±
0,216# |
3,6±
0,396* |
1,97±
0,155*∆ |
2,28±
0,139* |
3,18±
0,564* |
1,46±
0,094* |
| SOD, U/L | 381±
16,666 |
97±
0,638# |
192,71±
10,499* |
199,83±
14,747*∆ |
183,3±
8,227* |
110±
11,782 |
121,75±
5,045 |
Note: IM – a group of intact animals; NC – a group of animals of negative control; # – statistically significant relative to the IM group of mice (p<0.05, Newman-Keuls test); * – statistically significant relative to the NC group of animals (p<0.05, Newman-Keuls test); ∆ – statistically significant relative to the group of mice treated with levocabastine (p<0.05, Newman-Keuls test).
Evaluation of Changes in the Concentration of Markers of an Allergic Reaction (ELISA Study)
The results of the ELISA study are presented in table 2. In animals of the NC group under AR conditions, an increase in the concentration of histamine, IFN-γ, IL-6, IgE, and TNF-α was noted relative to the IM group of mice by 169.7% (p<0.05); 69.6% (p<0.05); 128.3% (p<0.05); 4.8 times (p<0.05) and 2.9 times (p<0.05), respectively.
The use of levocabastine contributed to the decrease in the serum levels of histamine, IL-6 and IgE by 33.9% (p<0.05); 23.3% (p<0.05) and 91.9% (p<0.05), respectively, while nasal administration of levocabastine did not significantly affect the change in the concentration of IFN-γ and TNF-α (there were no statistically significant differences with regard to NC group of animals is not established). Against the background of intranasal administration of beclomethasone to mice in relation to the NC group of animals, the concentration of proallergenic markers histamine, IFN-γ, IL-6, IgE, and TNF-α decreased by 56.3% (p<0.05); 49.5% (p<0.05); 79.3% (p<0.05); 234.1% (p<0.05) and 18.7% (p<0.05), respectively.
In terms of the introduction of the test-spray in a dose of 2.5 μg in mice compared with the NC group of animals, a decrease in the concentration of IL-6 by 49.2% (p<0.05), IgE by 157.9% (p<0.05) and TNF-α by 26.5% (p<0.05). At the same time, the use of the test spray at a dose of 7.5 μg resulted in a 48.3% decrease (relative to the NC group of mice) serum levels of histamine, IL-6, IgE and TNF-α (p<0.05); 25.9% (p<0.05); 210.9% (p<0.05) and 32.7% (p<0.05), respectively. On the background of the introduction of the test-spray in a dose of 5 μg in comparison with the NC group of animals, a decrease in the concentration of histamine, IFN-γ, IL-6, IgE, and TNF-α was observed by 69.1% (p<0.05); 38.1% (p<0.05); 130.8% (p<0.05); 248.2% (p<0.05) and 53.8% (p<0.05), respectively. At the same time, the content of histamine, IL-6, IgE, and TNF-α in mice that were injected with the test spray at a dose of 5 μg, was 26.2% (p<0.05); 81.2% (p<0.05); 81.4% (p<0.05) and 52.1% (p<0.05) are lower in relation to mice treated with levocabastine.
Table 2: Changes in the concentration of markers of an allergic reaction under the conditions of the correction of experimental allergic rhinitis with the test spray in various dose and reference drugs.
| Group | IM | NC | 2,5 μg | 5 μg | 7,5 μg | Levocabastine 5 μg | Beclomethasone 3,5 μg |
| Histamine, pg/ml | 20,57±
1,464 |
55,47±
4,126# |
53,23±
3,354 |
32,81±
0,955*∆ |
37,4±
3,587* |
41,42±
4,97* |
35,5±
4,291* |
| IFN-γ, pg/ml | 40,03±
1,103 |
68±
1,472# |
59,75±
1,315 |
49,25±
2,562* |
55,25±
5,072 |
52,5±
2,901 |
45,5±
1,848* |
| IL-6,
pg/ml |
15,84±
1,423 |
36,17±
1,952# |
24,25±
2,275* |
15,67±
1,504*∆ |
28,72±
0,665* |
29,33±
2,072* |
20,17±
1,363* |
| IgE,
pg/ml |
9,89±
1,313 |
47,04±
1,001# |
18,24±
1,452* |
13,51±
0,699*∆ |
15,13±
0,728* |
24,51±
0,329* |
14,08±
0,913* |
| TNF-α, pg/ml | 56,43±
1,322 |
165,84±
9,882# |
131,08±
2,434* |
107,8±
4,291*∆ |
124,94±
6,203* |
164,08±
14,497 |
139,62±
13,46* |
Note: IM – a group of intact animals; NC – a group of animals of negative control; # – statistically significant relative to the IM group of mice (p<0.05, Newman-Keuls test); * – statistically significant relative to the NC group of animals (p<0.05, Newman-Keuls test); ∆ – statistically significant relative to the group of mice treated with levocabastine (p<0.05, Newman-Keuls test).
Discussion
AR is a common pathology of the respiratory system with a high current incidence rate and unfavorable prognostic calculations that show a steady increase in the number of cases of AR in both economically developed and developing countries. In addition, AR is characterized by significant comorbidity with bronchial asthma, atopic dermatitis, sinusitis, which significantly reduces the quality of life of the population.20
Currently available strategies for pharmacotherapy of AR include both systemic and local effects on the human body. Often, AR therapy may be limited to hygienic measures aimed at eliminating causal factors (restriction of contact with the allergen, irrigation of the nasal mucosa with saline solutions). However, as a rule, these methods either do not have the desired effect, or their implementation is not possible (in some cases it is not possible to interrupt the patient’s contact with the allergen).21 In the case of inefficiency / impracticability of hygienic measures, the treatment of AR is carried out by pharmacotherapeutic methods. The basic principles of drug selection, for the purpose of AR therapy, are based on the optimal ratio of efficacy and safety of use, on this basis, systemic AR treatment is usually limited to oral administration of antihistamines, mainly selective antiallergic action (fexofenadine, desloratadine, levocetirizine), which are effective eliminate the main nasal symptoms of AR – sneezing, pruritus, rhinorrhea.22 However, the long latency of these agents does not allow the use of oral antihistamines, with due therapeutic effectiveness in the treatment of acute forms of AR.23 Local drug use is associated with a significantly lower toxic effect on the body, which allows the use of more effective drugs. Nasal glucocorticoids (mometasone, beclomethasone, budesonide, ciclesonide) can be attributed to this group of drugs, which have a pronounced therapeutic effect in all forms of AR occurring both chronically and acutely. However, given the fact of local use, glucocorticoid drugs are not devoid of a significant range of adverse reactions, including: nasal bleeding, dryness and burning in the nasal cavity, bacterial lesions of the nasal mucosa.24 In addition to antihistamines and glucocorticoid drugs, decongestants, hydrophilic M-anticholinergic drugs, and antileukotriene preparations are used in the treatment of AR, but these drugs have less therapeutic value compared to first-line drugs.25 To overcome the existing deficiencies of drugs of the H1-histamine receptor blockers and glucocorticoid groups, perhaps due to the rational combined use of the drugs of these groups. Currently available nasal spray “Dymista” (fluticasone propionate / azelastine), although it shows a sufficient level of therapeutic efficacy, but does not provide adequate safety of use, and the frequency and degree of manifestation of adverse drug reactions do not differ from those of intranasal glucocorticoids.26 Thus, the development of new safe means of local action for the treatment of AR, is of undoubted scientific and practical interest. In this regard, a study was conducted devoted to the study of the antiallergic action of the combined nasal spray containing fexofenadine hydrochloride and ammonium glycyrrhizinate. Fexofenadine hydrochloride is a highly effective H1-histaminolytic drug with a broad spectrum of antiallergic action – effective for the treatment of both acute and slow-moving chronic forms of allergic disease. It is important that fexofenadine has the optimal combination of high pharmacotherapeutic efficacy and safety of use – the incidence of adverse reactions when using fexofenadine is comparable to placebo.27 Ammonium glycyrrhizinate is a water-soluble derivative of glycyrrhizic acid, which has a broad spectrum of biological activity, including antiallergic action.28 Fundamental differences in the mechanisms of the antiallergic action of fexofenadine hydrochloride – an antihistamine drug and ammonium glycyrrhizinate, which have antiallergic effect mainly due to the restriction of transmembrane calcium current and stabilization of mast cell membranes, suggest the presence of synergistic interaction with potentiation of antiallergic action.
The study showed that the test-spray containing of fexofenadine hydrochloride + ammonium glycyrrhizinate in experimental allergic rhinitis exhibits a high level of pharmacological activity comparable to that of intranasal glucocorticoids (beclomethasone «Nasobec») and a superior effect monocomponent nasal aerodisperse systems antihistaminic action (levocabastine, «Tyzine ® Allergy»). The use of the test-spray in various dose made it possible to establish that the most optimal dose of the spray being studied is a dose of 5 μg, since the most significant reduction in nasal symptoms of AR in mice was noted during nasal instillation of the studied composition in this dose. In addition, the use of the studied composition at a dose of 5 μg in comparison with the antihistamine drug, levocabastine (Tyzine® Allergy), reduced the serum concentration of proallergic markers — histamine, IL-6, IgE and TNF-α. At the same time, no statistically significant differences between the groups of animals treated with the studied composition in a dose of 5 μg and beclomethasone were established. Against the background of the intranasal application of the test aerodisperse system, a decrease in the intensity of free-radical reactions was observed – one of the characteristic pathogenetic links of AR,29 which may indicate an increase in SOD activity and a decrease in the concentration of MDA under the conditions of application of the test-spray. It is worth noting that the restoration of pro / antioxidant balance was more pronounced when using the spray under study at a dose of 5 μg, which was superior to the comparison preparations in this action. Ultimately, the leveling of the leading pro-allergenic factors (hyperproduction of cytokines and free radicals) against the background of the introdution of mice of the test-spray contributed to the reduction of nasal symptoms of AR. When using the estimated spray in a dose of 5 μg compared with a group of animals without pharmacological support, a decrease in the number of acts of sneezing and paranasal grooming, compared to mice treated with levocabastine, a decrease in these indicators was observed. Thus, based on the results obtained in the course of this study, it can be assumed that the test nasal aerodisperse system at a dose of 5 μg has equivalent therapeutic potential with the reference drug beclomethasone («Nasobec») and exceeds the anti-allergic effect of the antihistamine drug levocabastine («Tyzine® Allergy»). It can be assumed that the test-spray has a low toxicity of use, as it contains components that have an optimal safety profile of use.
Conclusions
The study found that experimental OVA-induced allergic rhinitis in mice is accompanied by an increase (relative to intact animals) of the serum concentration of pro-allergic markers of histamine, IFN-γ, IL-6, IgE, and TNF-α, with the development of typical nasal symptoms and oxidative stress. Intranasal instillation of levocabastine («Tyzine® Allergy») at a dose of 5 μg / nostril and to a greater extent beclomethasone («Nasobec») at a dose of 3.5 μg / nostril contributed to the reduction of nasal symptoms of allergic rhinitis, a decrease in the concentration of proallergenic factors: histamine, IFN-γ, IL-6, IgE, and TNF-α and lipooxidation processes (decrease of MDA concentration and increase of SOD activity). . The use of the test-spray (fexofenadine hydrochloride + ammonium glycyrrhizinate) in the dose range of 2.5 μg; 5 μg and 7, 5 μg / nostril promoted to reduce the severity of the allergic reaction in mice as evidenced by the decrease of concentration histamine, IFN-γ, IL-6, IgE, TNF-α and oxidative stress markers (the concentration of MDA decreased, and the SOD activity increased). At the same time, the studied aerodisperse system at a dose of 5 μg had a comparable pharmacological effect with beclomethasone («Nasobeс») and exceeded in comparison with the therapeutic effect the comparison drug levocabastine («Tyzine® Allergy») and the test-spray at doses of 2.5 μg and 7.5 μg.
References
- Sinha B., Vibha., Singla R., Chowdhury R. Allergic Rhinitis: A neglected disease – A community based assessment among adults in Delhi. J Postgrad Med. 2015;61(3):169-75.
CrossRef - Dykewicz M. S., Hamilos D. L. Rhinitis and sinusitis. J Allergy Clin Immunol. 2010;125:103–S115. doi: 10.1016/j.jaci.2009.12.989.
CrossRef - World Health Organization [internet]. Global Surveillance, prevention and control of Chronic Respiratory Diseases: A Comprehensive approach 2007. [Lase accessed on 2018 Dec 22]. Available from:http://www.who.int/gard/publications/GARD%20Book%202007.pdf.
- Poddighe D., Licari A., Caimmi S., Marseglia G. L. Sublingual immunotherapy for pediatric allergic rhinitis: The clinical evidence. World J Clin Pediatr. 2016;5(1):47-56. doi: 10.5409/wjcp. v5.i1.47.
- Kim S. H., Won H. K., Moon S. D. Impact of self-reported symptoms of allergic rhinitis and asthma on sleep disordered breathing and sleep disturbances in the elderly with polysomnography study. PLoS One. 2017;12(2):e0173075. doi: 10.1371/journal. pone.0173075.
- Wilhelm C. P., deShazo R. D., Tamanna S., Ullah M. I., Skipworth L. B. The nose, upper airway, and obstructive sleep apnea. ANAI. 2015;115(2):96–102.
CrossRef - Wright R. J. Stress and atopic disorders. J Allergy Clin Immunol. 2005;116:1301–6.
CrossRef - Wheatley L. M., Togias A. Allergic rhinitis. N Engl J Med. 2015;372(5):456–63.
CrossRef - Prasad B., Nyenhuis S. M., Weaver T. E. Obstructive sleep apnea and asthma: associations and treatment implications. med. rev. 2014;18(2):165–71. doi: 10.1016/j.smrv. 2013.04.004.
- Small P., Keith P. K., Kim H. Allergic rhinitis. Allergy Asthma Clin Immunol. 2018;14(2):51. doi: 10.1186/s13223-018-0280-7.
CrossRef - Wang Q., Chen D., Xie H., et al. Altered Expression of IFN-λ2 in Allergic Airway Disorders and Identification of Its Cell Origins. Mediators Inflamm. 2016;2016:5759496.
CrossRef - Yanez A., Rodrigo G. J. Intranasal corticosteroids versus topical H1 receptor antagonists for the treatment of allergic rhinitis: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2002;89(5):479–484. doi: 10.1016/S1081-1206(10)62085-6.
CrossRef - Allen D. B., Meltzer E. O., Lemanske R. F. Jr., Philpot E. E., Faris M. A., Kral K. M., Prillaman B. A., Rickard K. A. No growth suppression in children treated with the maximum recommended dose of fluticasone propionate aqueous nasal spray for one year. Allergy Asthma Proc. 2002;23(6):407–413.
- Lee T. A., Pickard A. S. Meta-analysis of azelastine nasal spray for the treatment of allergic rhinitis. Pharmacotherapy. 2007;27:852–9.
CrossRef - Mösges R., Spaeth J., Klimek L. Efficacy and tolerability of levocabastine and azelastine nasal sprays for the treatment of allergic rhinitis. Mediators Inflamm. 1995;4(7):S11-5.
CrossRef - Li Q., Zhang Y. D., Sun C. W., Chen Y. L., Du Y. H., Zhao G. J & Zhang D. L. Treatment of allergic rhinitis rats by intranasal interferon gamma. Chinese j. otorhinol. head neck surg. 2008;43(2):134-138.
- Tonelli L. H., Katz M., Kovacsics C. E., et al. Allergic rhinitis induces anxiety-like behavior and altered social interaction in rodents. Brain Behav Immun. 2009;23(6):784-93.
CrossRef - Woolliams J. A., Wiener G., Anderson P. H., McMurray C. H. Vet. Sci. 1983;34:253-256.
- Stalnaya I. D., Garishvili T. G. Method for the determination of malondialdehyde using TBA. meth. biochem. 1977:44-46.
CrossRef - Rudack C. Actual therapeutic management of allergic and hyperreactive nasal disorders. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2004;3: Doc04.
- Small P., Frenkiel S., Becker A., et.al. The Canadian Rhinitis Working Group Rhinitis: a practical and comprehensive approach to assessment and therapy. J Otolaryngol. 2007;36(1):25–27. doi: 10.2310/7070. 2006.X002.
- Recto M. T., Gabriel M. T., Kulthanan K., et al. Selecting optimal second-generation antihistamines for allergic rhinitis and urticaria in Asia. Clin Mol Allergy. 2017;15:19. Published 2017 Nov 1. doi: 10.1186/s12948-017-0074-3.
- Kim H., Kaplan A. Treatment and management of allergic rhinitis [feature]. Clin Focus. 2008;1–4.
- Sahin A. A. Y., Corey J. P. Rhinitis in the elderly. Curr Allergy Asthma Rep. 2006;6:125–131. doi: 10.1007/s11882-006-0050-3.
CrossRef - Bozek A. Pharmacological Management of Allergic Rhinitis in the Elderly. Drugs Aging. 2016;34(1):21-28.
CrossRef - Berger W. E., Shah S., Lieberman P., Hadley J., Price D., Munzel U., Bhatia S. Long-term, randomized safety study of MP29-02 (a novel intranasal formulation of azelastine hydrochloride and fluticasone propionate in an advanced delivery system) in subjects with chronic rhinitis. J Allergy Clin Immunol Pract. 2014;2(2):179–185. doi: 10.1016/j.jaip. 2013.09.019.
- Axelrod D., Bielory L. Fexofenadine hydrochloride in the treatment of allergic disease: a review. J Asthma Allergy. 2008;1:19-29. Published 2008 Sep 19.
- Han S., Sun L., He F., Che H. Anti-allergic activity of glycyrrhizic acid on IgE-mediated allergic reaction by regulation of allergy-related immune cells. Sci Rep. 2017;7(1):7222. doi:10.1038/s41598-017-07833-1.
CrossRef - Chauhan B., Gupta M., Chauhan K. Role of antioxidants on the clinical outcome of patients with perennial allergic rhinitis. Allergy Rhinol (Providence). 2016;7(2):74-81.
CrossRef








