Anak Agung Gde Putra Wiraguna*1, Rini Dianasari2 and Wimpie Pangkahila3
1Dematology and Venereology Department, Faculty of Medicine, Udayana University-Sanglah General Hospital, Bali-Indonesia.
2Biomedicine Magister Program, Post Graduate Program, Faculty of Medicine, Udayana University.
3Anti Aging Medicine Department, Faculty of Medicine, Udayana University.
Corresponding Author E-mail: wiraguna@unud.ac.id
DOI : https://dx.doi.org/10.13005/bpj/1641
Abstract
Formation of free radicals is an important mechanism causing skin aging. Free radicals are highly reactive molecules with unpaired electrons which can directly disrupt various structures of cell membrane, lipids, proteins, and DNA. Antioxidant is a substance which can give protection from endogenous and exogenous oxidative pressure caused by free radicals. Purple corn contains phenolic acid, vitamin C, and anthocyanin. Anthocyanin is the main contained substance in purple corn and acts as antioxidant and able to inhibit aging process on skin surface of mice exposed with UV-B ray. This research aims to prove the effectivity of administration of purple corn extract cream on inhibiting the elevation of MMP-1 level and the decrease of collagen amount on Wistar mice (Rattus norvegicus) exposed with UV-B. This research is animal experiment with post test only control group design. As many as 36 mice were divided into 2 groups containing 18 mice each, control group with appliance of placebo cream and intervention group applied with 50% purple corn extract cream. All groups were exposed with UV-B with dose of 840 mJ/cm² for 4 weeks, and biopsy was taken to examine the level of MMP-1 and collagen amount on dermis. The results of Shapiro-Wilk and Levene’s test showed that the data distribution between the two groups was normally distributed with homogenous variance and p ≥ 0.05. Comparative analysis with t-independent test showed that there is a significant difference between both groups, either on the mean level of MMP-1 or the collagen amount on both groups with p < 0.05. The mean collagen amount and mean MMP-1 level of intervention 2 are 71.7% and 1.9 mg/ml, respectively. Intervention-1 group shows the mean amount of collagen and MMP-1 level are 65.54 % and 3.22 mg/ml, respectively. The conclusion of this research is the administration of 50% purple corn extract cream inhibits the increase of MMP-1 level and the decrease of the amount of dermal collagen on mice’s skin exposed with UV-B.
Keywords
Anthocyanin; Dermal Collagen Amount; 50% Purple Corn Extract Cream; MMP-1 Level; UV-B Ray
Download this article as:| Copy the following to cite this article: Wiraguna A. A. G. P, Dianasari R, Pangkahila W. The Topical Skin Application of Purple Corn Extract (Zea Mays) Inhibited the Increase in MMP-1 Levels and Decreased Collagen in Wistar Rats (Rattus Norvegicus) Exposed to UV-B Rays. Biomed Pharmacol J 2019;12(1). |
| Copy the following to cite this URL: Wiraguna A. A. G. P, Dianasari R, Pangkahila W. The Topical Skin Application of Purple Corn Extract (Zea Mays) Inhibited the Increase in MMP-1 Levels and Decreased Collagen in Wistar Rats (Rattus Norvegicus) Exposed to UV-B Rays. Biomed Pharmacol J 2019;12(1). Available from: https://bit.ly/2tLnfTS |
Introduction
Aging or aging process is a natural process that will be experienced by every living thing in this world, but the aging process of everyone is not the same, there are some people who experience the aging process faster than others. The speed of the aging process is influenced by several factors, both extrinsic and intrinsic factors.
The process of intrinsic aging is an aging process that takes place naturally due to various factors in the body itself, such as genetic, hormonal, and racial. Extrinsic aging process occurs due to various factors from outside the body such as sun / ultraviolet light, humidity, temperature, cigarette smoke, pollution, and various other external factors that can accelerate the aging process so that premature aging occurs. This process can be prevented by avoiding the factors that accelerate the process.1-3
At the age of aging, there will be a decrease in function and ability to adapt to the occurrence of damage in the body. Also accompanied by a decline in various organs of the body and physical changes at the cellular level and in the system due to the aging process.4 Many factors play a role in the occurrence of the process, which can be grouped into internal factors and external factors. Internal factors include the presence of free radicals, reduced hormones, the process of glycosylation, apoptosis, decreased immune system and genes. External factors include unhealthy diet, unhealthy lifestyles, wrong habits, environmental pollution, radiation, UV rays, cigarette smoke, and stress.5
The aging process is the result of the interaction of the genetic program and the cumulation of the process of wear and tear throughout life.6 Similar to other organs in the human body, the skin also experiences aging, both internally and externally as mentioned above. In addition, the skin is an organ that has direct contact with the environment so that it is very affected by environmental factors such as ultraviolet (UV) sunlight.
Aging caused by chronic UV radiation from sunlight is referred to as photoaging, which is aging that occurs due to chronic adverse effects of sunlight accumulating with chronological aging symptoms. This process is cumulative chronic reactions to exposure to the sun’s ultraviolet rays for years can cause skin architectural disturbances, and especially cause premature aging of the skin (photoaging), and skin cancer.6, 7
Damage caused can be seen both clinically, histologically or anatomically or functionally. Exposure to UV radiation to sunlight causes skin damage through several mechanisms, including the formation of sunburn cells, the onset of inflammatory response, the formation of thymine dimers and collagenase production (MMP / Metalloproteinase Matrix).9 MMP is a proteinase enzyme containing zinc, which is responsible for degrading extracellular matrix proteins. MMP is classified as collagenase, gelatinase, stromelysin and membrane type.
UV radiation is known to directly and indirectly interfere with extracellular integrity of the matrix by increasing MMP activity. In human skin, MMP-1 is the type most affected by sun UV induction and is responsible for the breakdown of collagen in the skin undergoing photoaging.10 It was found that only one exposure to UV radiation can interfere with the connective tissue by causing synthesis disorders collagen is almost complete, 24 hours later followed by recovery 48-72 hours later.10 In addition, collagen degradation also occurred due to significant increase in MMP-1 levels which was around 4.4 ± 0.2 times compared to skin that is not exposed to UV radiation.10 MMP-1 is the main mediator for the onset of collagen degradation in the skin undergoing photoaging. Collagenolytic MMP-1 enzyme degrades collagen and elastin fibrils, which are important for skin strength and elasticity. MMP-1 activity in the skin will increase even with only short UV radiation, which will cause wrinkles on the skin, which is a sign of photoaging.11 Thus, inhibition of MMP-1 is one way to prevent skin damage from UV exposure.
In addition, ultraviolet radiation produces reactive oxygen species (ROS), along with the activation of various ROS-sensitive signaling pathways, which in turn affects various types of cellular functions including causing collagen fragmentation and MMP-1 secretion. Oxidative stress has a major effect in the process of photoaging and photocarcinogenesis and also in the pathogenesis of photodermatosis. 13
Antioxidants are known to prevent and counteract the formation of free radicals.11,13 The use of chemicals that function to protect the skin from the dangers of solar radiation has been widely used. One of them is polyphenol compounds from plants. The use of this material is intended to prevent, restore, and slow the adverse effects of UV radiation on the skin. The photoprotective effect of the skin from polyphenol material seems to be obtained from its ability as an anti-inflammatory, antioxidant, and DNA repair mechanism.14
Polyphenols are a group of chemicals (phytochemicals) found in plants, characterized by the presence of more than one phenol unit per molecule. Phenolic in human food consists of phenolic acid, tannin, and flavonoids. The most widely studied polyphenols are flavonoids, which are divided into two major groups, anthocyanins and anthoxanthins. Anthocyanin is a very important water-soluble pigment, which is responsible for giving red, blue, and purple colors to plants.15 These pigments are widely found in our food, including fruits such as blueberries, cranberries, bilberries, also in eggplant, brown rice, grape skin, purple yam, and purple corn.
Anthocyanin has been widely used throughout the world as food coloring, and since ancient times has been widely used as an herbal medicine that can cure hypertension, fever, liver disorders, diarrhea and dysentery, urinary disorders and influenza. Several previous studies have shown that anthocyanins have high potential bioactivity in the prevention of various chronic diseases such as diabetes and cataracts triggered by diabetes.16 Anthocyanins can also improve blood lipid profiles and have vasoprotective effects and also have an effect on inhibiting growth and stimulating cell apoptosis cancer.17
Purple corn (Zea Mays L.) has been widely cultivated in South America, especially in Peru and Bolivia, and is used to prepare drinks and desserts for centuries due to its high pigment content. Purple corn contains high concentrations of anthocyanin (~ 1640 mg / 100g Weight) much higher than other anthocyanin-rich sources, such as berries (20 ~ 1500 mg / 100g Weight), turnips (Raphanus sativus L.) (11 ~ 60 mg / 100g Weight), and cabbage (Brassica oleracea L.) (322 mg / 100g Weight). Interest in purple corn as a source of anthocyanins as color and phytonutrients have increased over the past year. Many health benefits have been attributed to purple corn, including reduced oxidative stress, prevention of obesity and diabetes, and colon cancer.18
In the study of Wistar rats exposed to UV-B rays and then smeared with purple corn extract cream at a dose of 25%, 50%, 100% proved that purple corn extract with a 50% dose had a protective effect on the skin of Wistar rats by increasing the amount of dermis collagen and reduce MMP-1 levels. Considering this fact, there is a suspicion that anthocyanins which are widely found in purple corn extracts can inhibit premature aging of the skin, by inhibiting the increase in MMP-1 levels in rats exposed to UV-B rays, due to their antioxidant effects. So it is necessary to do research to prove the allegations above.
Method
This study uses an experimental design with post test only control group design. This study was conducted in vivo, using 36 Wistar rats aged 10-12 weeks, male sex and body weight between 150-160 g, randomly grouped into 2 groups and each group consisted of 18 rats. Group 1 (Control Group) was the group given the basic ingredient of the placebo cream (basic cream only), group 2 (Intervention Group) was the group given the cream of purple corn extract (Zea mays L.). Group 1 and group 2 besides being given cream were also exposed to UV-B rays.
Research Parameters
The amount of dermis collagen in the skin of Wistar rats, if there is an increase is a sign of the protective effect of purple corn extract (Zea mays) on collagen damage due to exposure to UV-B rays and biomarkers of increased fibroblast production. The MMP-1 level of Wistar rats’ skin, if there is an increase is a sign of oxidative stress, and if decreased is a sign of the protective effect of purple corn extract (Zea mays) on collagen damage due to UV-B exposure.
Rat back skin tissue is taken 48 hours after irradiation, made 2×2 cm pieces from the skin of the rat’s back. The tissue obtained from the skin of the rat back will then be standardized using a collagen amount calculated by the method of digital analysis, each preparation of preparations was photographed using LC Evolution camera and Olympus Bx51 microscope with objective magnification 40 times, each preparation was photographed 3 times stored in JPEG format. MMP-1 level was examined using ELISA method.
Purple Corn Extract and UV-B Exposure
Purple corn extract is extracted from purple corn which is made using ethanol solvent, then concentrated with Danke and Kunkel rotary evaporator vacuum, IKA Labortechnik RV 06-ML, so that crude extract is obtained at the Agricultural Technology Laboratory, Faculty of Agricultural Technology Udayana University.
The selected purple corn is fresh with the following criteria: length of approximately 20 cm, middle diameter of cob 5 cm, and number of cobs 16 – 18 rows, weight 400 grams, purple-black color, non-defect, no rot, no insects and dirt. Purple corn used is corn that is suitable for consumption.
The cream base, 50% purple corn cream was applied twice a day, 20 minutes before irradiation (to give the absorption time of the topical material into the skin) and 4 hours after irradiation (ROS formation starts 4 hours after exposure). Application of topical ingredients is still done on days without irradiation.
Chronic UV-B exposure is given to groups 1 and 2, exposure is done 3 times a week starting with 50 mJ / cm2 in the first week, followed by 70 mJ / cm2 in the second week, and the next 2 weeks with 80 mJ / cm2 so that total UV B received by each group of rats was 840 mJ / cm2 for 4 weeks.
Histological Collagen Examination
48 hours after the last irradiation, to get rid of the effects of acute irradiation, all rats from the five groups were decapitated, then their 2×2 cm back skin tissue was taken, prior to skin dissection all animal were anaesthetized. The rat skin tissue was immersed in a 10% phosphate buffer formalin solution for 1 day (24 hours). Then trimming the part of the network that will be taken. The rat skin tissue was soaked with multilevel alcohols, respectively 50%, 70%, 90%, 96% and 100% for 2 hours, respectively. The tissue is inserted into the clearing agent (xylene) for 24 hours until it is transparent. Beginning with the infiltration process 2 times for each 1 hour with pure paraffin (Histoplast) liquid (temperature 60o C) then the tissue is planted into liquid paraffin and allowed to form a block that takes one day, to be easily sliced with microtomes. Cutting using a rotary microtome (Jung Histocut Leica 820), 5 micro meters thick in series and taking slices to 5, 10, 15 for subsequent attachment to the glass object, then incubated at 60°C celcius for 2 hours. The amount of dermis collagen is the percentage of collagen network pixels in the form of bright red tissue with Sirius red staining compared to the entire network pixel seen in histological preparations photos and expressed in percent (%). The assessment was carried out on photos of preparations in JPEG format taken with LC Evolution cameras and Olympus Bx51 microscopes with 40 times objective magnification, each preparation was photographed 3 times.
Statistic Analysis
Statistical analysis using SPSS version 20.0 for Windows, the test used was the average comparative analysis by independent sample t-test to compare collagen mean and MMP-1 levels of mouse skin exposed to UV-B rays.
Result
Experimental study with Randomized Post Test Only Control Group Design, using 36 healthy male Wistar rats weighing 150-160 grams and aged 10-12 weeks, which were divided into 2 (two) groups, namely the control group (exposed to UV-B light + topical placebo) and intervention group (exposed to UV-B light + topical 50% purple corn extract cream). There were no rats that experienced drop out (death) during the research process.
Treatment effect analysis was tested based on the mean MMP-1 level between the control group and the intervention group. The results of the significance analysis with the t-independent test are presented in Table 1.
Table 1: Comparison of MMP-1 levels between the control group and the intervention group.
| Study group | n | MMP-1 level (mg/ml) | SD | t | p |
| Control group | 18 | 3.22 | 0.47 | 5.71 | |
| Intervention group | 18 | 1.9 | 0.86 | 0.001 |
In Table 1, it shows that the mean of MMP-1 control group was 3.22 ± 0.47 and the mean group of purple corn extract cream was 1.90 ± 0.86. Analysis with t-independent test shows that the value of t = 5.71 and p = 0.001. This means that the MMP-1 mean in both groups after being given treatment differed significantly (p <0.05).
Treatment effect analysis was tested based on the number of collagen between groups after being given a 50% purple corn extract cream. The results of the significance analysis with the t-independent test are presented in Table 2.
Table 2: Differences in Average Number of Collagen Between Groups After 50% Purple Corn Extract Cream.
| Study group | n | Mean Collagen (%) | SD | t | p |
| Control group | 18 | 65.54 | 5.61 | 3.44 | 0.002 |
| Intervention group | 18 | 71.70 | 5.11 |
Table 2 shows that the control group’s average collagen was 65.54 ± 5.61 and the mean group of purple corn extract cream was 71.70 ± 5.11. Analysis with t-independent test showed that the value of t = 3.44 and the value of p = 0.002. This means that the average number of collagen in the two groups after being treated was significantly different (p <0.05).
Histological preparations in the control group show damage to the composition and structure of the red collagen tissue that appears thin. The black and yellow arrow indicates that the collagen fibers are not intact (Figure 1A). Histological preparations in the intervention group amount of collagen with red collagen fibers appear wider and thicker where intact collagen fibers begin to appear. Black and yellow arrows show intact collagen fibers (Figure 1B).
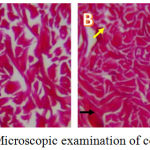 |
Figure 1: Microscopic examination of collagen tissue.
|
Discussion
Matrix Metalloproteinase 1 (MMP-1)
In this study, administration of UV-B light exposure in the control group for four weeks with a total dose of 840 mJ / cm2 causes an increase in the levels of MMP-1 significantly compared with intervention group. The mean MMP-1 control group 1 is 3.22 ± 0.47 and mean of MMP-1 in intervention group given purple corn extract cream was 1.90 1 ± 0.86) (Table 1).
Matrix metalloproteinases are a zinc-dependent endopeptidase, an enzyme responsible for the degradation of connective tissue of the dermis. Matrix metalloproteinases are involved in a wide range of proteolytic activity in both the physiological and pathological circumstances such as embryogenesis, wound healing, inflammation, angiogenesis, and matrix metalloproteinases in skin cancer.18 most of its formation is triggered by exposure to UV light is MMP-1 and most responsible for solving collagen. Besides the UV exposure, the levels of MMP-1 also increases with age, this will lead to fragmentation and disorganization of the arrangement of collagen fibers in the dermis.10
Exposure to UV light on the skin, will cause oxidative stress and this will activate cytokine receptors and growth factors on the surface of epidermal keratinocytes and fibroblast cells in the dermis. Activation of these receptors will induce intracellular signals of MAP Kinase which then activates the transcription factor AP-1. Activator protein -1 (AP-1) which is a nuclear transcription factor, consists of two sub-units, namely c-jun and c-fos, which functions to control MMP transcription.19
Nuclear factor kappa B (NF-kB) and activator protein-1 (AP-1) are transcription factors that are regulated by cellular redox conditions, and are involved in the regulation of gene expression. Both transcription factors are responsible for regulating various extracellular signaling molecules involved in inflammatory processes, cell proliferation, apoptosis, tumorigenesis, and tissue repair. These two transcription factors are very important in degenerative processes caused by UV exposure associated with photoaging such as induction of matrix metalloproteinases, and both are targets of anti-aging preventive therapy.20 The metalloproteinase-1 matrix, MMP-3, and MMP-9 are the most increased levels after exposure to UV-B rays. MMP-1 and MMP-3 mRNA increases nearly 1000 times after 24 hours of UV exposure.18 After collagen is broken down by MMP-1, collagen is further degraded by increasing MMP-3 and MMP-9.10,18
Dermal fibroblasts are the main source of MMP-1 and increase after exposure to UV-B rays in both cell culture and skin cells in vivo.21 The matrix metalloproteinase-1, MMP-3, and MMP-9 are initially produced in the epidermis, but these enzymes can diffuse into the dermis and then degrade collagen.18 This diffusion is also aided by direct bonding of MMP to extracellular matrix collagen. Although there are studies that suggest that keratinocytes are the main source of MMP, they are produced in response to the skin’s exposure to UV-B rays but it is possible that dermis fibroblasts also play a role in keratinocyte MMP production through indirect paracrine mechanisms by releasing growth factors and cytokines. triggers MMP production by keratinocytes.10,18
UV-B exposure with a total dose of 840 mJ / cm2 for four weeks was able to increase MMP-1 levels in mouse skin tissue.22 After being given topical purple corn extract cream, MMP-1 levels decreased. This proves that these compounds have reduced activity against free radicals.
The ability of purple corn extract to reduce the levels of MMP-1 dermis tissue of Wistar rats was played by various active substances contained in it, including vitamin C, anthocyanins and polyphenols. Polyphenols are powerful antioxidants, in several in vitro studies proven antioxidant activity is stronger than vitamin C, E, and carotenoids. The protective effects of fruits and vegetables in reducing the risk of diseases associated with oxidative stress such as heart disease, cancer or osteoporosis are partly thought to come from polyphenols. The antioxidant effects of phenolic compounds in the body can be through three mechanisms such as reducing free radicals, suppressing the formation of free radicals by inhibiting several enzymes or chelating trace metals involved in the production of free radicals, and increasing the supply of antioxidants or protecting antioxidant defenses.10,18
The ability of 50% purple corn extract cream to reduce MMP-1 levels is likely because the active substance contained in purple corn extract works synergistically to increase its antioxidant capacity. High anthocyanin content in purple corn (~ 1640 mg / 100g Weight) as an antioxidant effect also has anti-inflammatory effects, anti-diabetic, anti-cancer effects, and can improve blood lipid profiles and have vasoprotective effects.24
The phenolic structure of anthocyanin is responsible for its antioxidant effects, namely the hydroxyl group at position 3 of ring C and position 3 ‘, 4’, 5 ‘of the ring B. As an antioxidant, anthocyanins work as ROS scavenger such as superoxides (O2–), singlets oxygen (‘O2), peroxide (ROO–), hydrogen peroxide (H2O2) and hydroxyl radical (OH–).25
Number of Collagen
The control group’s average collagen was 65.54 ± 5.61 and the mean of collagen in intervention group 71.70 ± 5.11 (Table 2). The decrease in dermal tissue collagen expression in control group is a sign of oxidative stress due to excessive free radical formation.26 Oxygen molecules (O2) in the lower part of the epidermis are the main targets of UV-B light entering the skin. UV light that penetrates the skin can be a donor of an oxygen molecule that causes oxygen to become unstable, becoming an aggressive free radical (superoxide anion).
This superoxide (O2–) anions will randomly take an electron from the nearest molecule and not only damage the molecule, but also turn it into free radicals, and this causes a chain reaction. This type of formation or spread of free radicals can damage various components in the skin, such as enzymes and cell membranes. The second electron derived from UV-B light can be given to superoxide anions, by forming hydrogen peroxide (H2O2). Hydrogen peroxide can also be converted to hydroxyl radical (OH–) in the presence of iron (Fe2+) through the Fenton reaction. Hydroxyl radicals are a very dangerous threat to cells, because these free radicals can enter through the core membrane and damage DNA. H2O2 and OH levels can be detected within 15 minutes after UV exposure and continue for up to 60 minutes.10
It is reported that ultraviolet radiation damages the skin collagen matrix through two different pathways, the stimulation of collagen degradation and inhibition of collagen production.10,18 When the skin is exposed to sunlight, UV radiation is absorbed by skin molecules that can cause harmful compounds called reactive oxygen species (ROS).10 Which can cause oxidative damage to cell components such as cell walls, lipid membranes, mitochondria, and DNA. This ROS also has a large effect on molecular pathways. Radiation of human buttocks skin with 2 MED (minimum erythema dose, ie a minimal dose of UV-A / UV-B radiation that can cause erythema effects on the skin) can increase hydrogen peroxide in 15 minutes.19
While other studies found that with only one exposure to UV radiation exposure of 4 MED, it could induce markers of oxidative stress on the skin (epidermis and dermis), H2O2 (using dihydrorhodamine-123, DHR), nitric oxide (using spectrophotometry), lipid peroxidation (using Malondialdehyde, MDA), and infiltration of inflammatory leukocytes (using CD11b + cell antibodies) which increased most significantly at 48 hours after UV exposure. As is known that the presence of nitric oxide (NO) and hydrogen peroxide (H2O2) is very damaging and cytotoxic to target cells. NO contains unpaired electrons and is paramagnetic, and therefore reacts quickly to superoxide anions to form peroxy nitrate anions. The decomposition of peroxy nitrate is a strong oxidant, just like a hydroxyl radical.27
In addition, UV radiation can cause changes in dermal collagen in two ways: (1) stimulation of the breakdown of collagen, resulting in collagen that is fragmented in fragments and irregular, (2) inhibits procollagen biosynthesis, so that collagen content decreases.11 Only one UV radiation with a dose of 2 MED can inhibit almost total procollagen synthesis, which lasts for 24 hours, followed by repair in 48-72 hours after that.10 Previous research has also found that AP-1 (Activator protein-1) and MMP are elevated and persist for about 24 hours after exposure to UV radiation and there is a significant increase in collagen breakdown.18
Conclusion
Topical administration of 50% Purple Corn extract cream inhibited the increase of MMP-1 levels and inhibited the decrease in collagen tissue amount of dermis of Wistar rat skin exposed to UV-B rays.
Ethical Statement
The research ethics committee of Faculty of Medicine Udayana University has approved current study with reference number 545/UN.14.2/Litbang/2014.
Conflict of Interest
Author declare no conflict of interest regarding all elements in this study.
Funding
This research doesn’t receive any specific funding from private sector or government organization.
References
- Wlascheck I., Tantcheva-Poor L., Naderi W., Ma S., Alexander Z., Razi-Wolf .J, Shuller K., Scharffetter K. Solar UV irradiation and dermal photoaging. Journal of Photochemistry and Photobiology. 2001;63:41–51.
CrossRef - Baran R., Maibach H. I. Editor. Texbook of Cosmetic and Dermatology 3rd Abingdon: Taylor & Francis Group. 2005: 445-54.
- Yaar M., Gilchrest B. A., editor. Biochemical and Molecular Changes in Photoaged Skin. San Fransisco: Blackwell Science. 2008:168-179.
- Baumann L. Cosmetic and Skin Care in Dermatology. Philadelphia: Mc graw-Hill Book co. 2005:2363-2367.
- Rabe J. H., Mamelak A. J., Mc Elgunn P., Morison W. L., Sauder D. N. Photoaging: Mechanism and Repair, Continuing Medical Education, American Academy of Dermatology, Inc. 2006:1-19.
- Gilchrest B. A., Yaar M. Aging of Skin. Philadelphia: Mc Graw-Hill Book Co. 2000:1386-1387.
- Krutmann J and Glichrest B. A. Photoaging of Skin. Heidenberg: Springer. 2006:33-43.
- Berneburg M., Plettenberg H., Krutmann J. Photoaging of Human Skin. Photodermatology, Photoimunology, dan Photomedicine. 2000;16:239-244.
CrossRef - Baumann L. Cosmetics and Skin Care in Dermatology. New York: McGrawHill. 2008:2357-63.
- Fisher G. J., Kang S., Varani J., Csorgo Z. B., Wan Y., Datta S., Voorhees J. J. Mechanism of Photoaging and Chronological Skin Aging. Arch. Dermatol. 2001;38:1462-1470.
- Yaar M., Gilchrest B. A. Biochemical and Moleculer Changes in Photoaged Skin. Philadelphia: Blackwell Science. 2008:168-179.
- Lee Y. R., Noh E. M., Jeong E. Y., Yun E. K., Kim J. H., Kwon K. B., Kim B. S., Lee S. H., Park C., Kim J. S. Cordycepin Inhibits UV-B-Induced Matrix Metalloproteinase Expression by Suppressing the NFκB Pathway in Human Dermal Fibroblast. Experimental and Molecular Biomedicine. 2009; 41:548-554.
CrossRef - Stahl W., Heinrich U., Wiseman S., Eichler O., Sies H and Tronnier H. Dietary Tomato Paste Protects against Ultraviolet Light-Induced Erythema in Humans. J. Nutr. 2001;131:1449–51.
CrossRef - Nichols J. A., Katiyar K. Skin Photoprotection by Natural Polyphenols: Anti-inflammatory, Antioxidant and DNA Repair Mechanisms. Arch. Dermatol. Res. 2010;302(2):1-19.
CrossRef - Fuhrman B., Aviram M., editors. Handbook of Antioxidants 2nd New York: Marcel Dekker Inc. 2002:306-311.
- Ghosh D., Konishi T. Anthocyanin and Anthocyanin-rich extract: role in diabetes and eye function. Asia. Pac. J. Clin. Nutr. 2007;16(2):200-208.
- Astadi I. R., Astuti U., Santoso P. S. In vitro antioxidant activity of anthocyanins of black soybean seed coat in human low-density lipoprotein (LDL). Food. 2009;122:659-663.
CrossRef - Wang J., Lian W., Cao Y., Wang X., Wang G., Qi C., Liu L., et al. Overexpression of BoNAC019, a NAC transcription factor from Brassica oleracea, negatively regulates the dehydration response and anthocyanin biosynthesis in Arabidopsis. Sci. Rep. 2018;8(1):13349.
CrossRef - Chen Y., Hung Y. C., Chen M., Lin M., Lin H. Enhanced storability of blueberries by acidic electrolyzed oxidizing water application may mediate by regulating ROS metabolism. Food Chem. 2019;270:229-235.
CrossRef - Xu Z., Mahmood K., Rothstein S. J. ROS induce anthocyanin production via late biosynthetic genes and anthocyanin deficiency confers the hypersensitivity to ROS-generating stresses in Arabidopsis. Plant Cell Physiol. 2017;58(8):1363-1377.
CrossRef - Islam M. S., Giampieri F., Janjusevic M., Gasparrini M., Forbes-Hernandez T. Y., Mazzoni L., Greco S., et al. An anthocyanin-rich strawberry extract induces apoptosis and ROS while decrease glycolysis and fibrosis in human uterine leiomyoma Oncotargets. 2017;8(14):23575-23587.
- Xu Z., Rothstein S. J. ROS-induced anthocyanin production provides feedback protection by scavenging ROS and maintaining photosynthetic capacity in Arabidopsis. Plant Signal Behav. 2018;13(3):e1451708.
CrossRef - Sudheeran P. K., Feygenberg O., Maurer D., Alkan N. Improved cold tolerance of mango fruit with enhanced anthocyanin and flavonoid contents. Molecules. 2018;23(7):E1832.
CrossRef - Maleva M., Garmash E., Chukina N., Malec P., Waoloszek A., Strzalka K. Effect of the exogenous anthocyanin extract on key metabolic pathways and antioxidant status of Brqazilian elodea (Egeria densa (planch.) casp.) exposed to cadmium and manganese. Ecotoxicol. Environ. 2018;160:197-206.
CrossRef - Valenza A., Bonfanti C., Pasini M. E., Bellosta P. Anthocyanins function as anti-inflammatory agents in a drosphila model for adipose tissue macrophage infiltration. Biomed. Res. Int. 2018;64:13172.
- Huang W., Yan Z., Li D., Ma Y., Zhou J., Sui Z. Antioxidant and anti-inflammatory effect pf blueberry anthocyanins on high glucose-induced human retinal capillary endothelial cells. Oxid. Med. Cell. Longev. 2018;18:62462.
- Greul A. K., Grundman J. U., Heinrich F., Pfitzner I., Bernhardt J., Ambcah A., et al. Photoprotection of UV-irradiated human skin: an antioxidative combination of vitamins E and C, carotenoids, selenium and Skin Pharmacol. Appl. Skin Physiol. 2002;15(5):307-15.
CrossRef








