Manuscript accepted on :June 25, 2016
Published online on: --
Plagiarism Check: Yes
Bahram Fariborz Farsad1, Homan Bakhshandeh2 and Sama Mahboubi3*
1Pharm.D, BCPS, Assistant Professor, Rajaie Cardiovascular Medical and Research Center, Iran University of Medical sciences, Tehran, Iran.
2Head of Epidemiology Department, ShahidRajaei Heart Center, Tehran, Iran.
3Pharmacist, ShahidRajaei Heart Center, Tehran, Iran.
*Corresponding Author E-mail: samamahboobi@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/973
Abstract
Prophylactic intravenous antibiotics should be routinely administrated to patients undergoing cardiac surgery. After cardiac surgery the patients are routinely administrated with prophylactic intravenous antibiotics. The duration and doses of such antibiotics are still controversial and the wide spectrum of prophylactic antibiotics used across the planet. It is important to bear in mind the sensitivity of administrating post-surgical antibiotics, using guidelines for standard administration prescribing are either missing or not effectively treated in many countries. However antibiotic prophylaxis is not the only preventative measures. The most common antibiotics used in the management of CABG associated infections different antibiotics been investigated. Different generation of Cephalospurins are most important study groups of antibiotics. It is important to appreciate that this reduction is not refer as a primary goal for surgical prophylaxis. To compare the antibiotics prophylaxis utilization in ShahidRajaei Heart Center, Tehran, Iran, with international recognized standards, we conducted an observational 6-month study at this center, by a sectional, descriptive and prospective study.This review including the antibiotics administrated to the patients during their hospitalization in ICU as well as CCU in ShahidRajaei hospital. The data gathered was done on the bases of a questionnaire including the patients’ history and the administrated antibiotics. . Each case is independently compared with the respective guideline in order to study its consistency (in dose, duration and frequency) to globally-applied guideline, i.e. ASHP, ACC/AHA, NHS ESC etc. The collected data have been analyzed by SPSS software, in order to figure out the descriptive report. As the background characteristics, 130 patients (37 women, 93 men) with mean age of 64±8 years participated. Most of the Antibiotics prescriptions did not follow the guidelines in proper manner. The most prevalent Antibiotics utilized is this center for reduction of SSI after CABG, are Vancomycin, Ceftriaxone, Cefazolin and Ciprofloxacin. The most prominent problem in Antibiotics prophylaxis utilization in the target medical center is the non-compliance of selection and duration of antibiotics to the applicable guidelines.
Keywords
Drug Utilization; Prophylactic Antibiotics; CABG Patients
Download this article as:| Copy the following to cite this article: Farsad B. F, Bakhshandeh H, Mahboubi S. Drug Utilization and Evaluation of Prophylactic Antibiotics in CABG Patients. Biomed Pharmacol J 2016;9(2). |
| Copy the following to cite this URL: Farsad B. F, Bakhshandeh H, Mahboubi S. Drug Utilization and Evaluation of Prophylactic Antibiotics in CABG Patients. Biomed Pharmacol J 2016;9(2). Available from: http://biomedpharmajournal.org/?p=7622 |
Introduction
Prophylactic intravenous antibiotics should be routinely administrated to patients undergoing cardiac surgery.1 In comparison to other types of surgeries the risk of infection in cardiac surgery is higher because of some features such as cardiopulmonary bypass and systemic cooling for myocardial protection, remaining some devices after surgery (chest drains, pacing wires, intravenous catheters), higher risk of post-operative bleeding leading to blood transfusion, and delayed extubation.2After cardiac surgery the patients are routinely administrated with prophylactic intravenous antibiotics. The duration and doses of such antibiotics are still controversial and the wide spectrum of prophylactic antibiotics used across the planet. Currently the recommended antibiotics are beta lactams which are given 30 minutes or in the case of glycopeptides 60 minutes prior to the start of the surgery, However this recommendation a sometimes hard to follow, particularly in the cases of emergency. Incidentally the required intra-operative doses (for prolonged surgeries) or post- operative doses are sometimes omitted or delayed.1
Various systemic reviews investigating randomized clinical controlled trials been carried out to evaluate the use of antibiotic prophylaxis in cardiac surgeries. The most important considered criteria were type of surgery, the timing and duration of antibiotic prophylaxis, methods of surveillance of surgical site infection (SSI) and mortality. One of the important factors are the cost of antibiotics because they are costly drugs which have specific side effects, as well as the risk of development of resistant micro-organisms resulted by their extensive use.15 It is important to bear in mind the sensitivity of administrating post-surgical antibiotics, using guidelines for standard administration prescribing are either missing or not effectively treated in many countries.2,3However antibiotic prophylaxis is not the only preventative measures. Other measures such as proper aseptic surgical techniques, pre-operative screening for potential resistant organisms, pre-operative cleansing options and hair removal, perioperative skin preparation, temperature and blood glucose and maintenance of reasonable hemoglobin saturation and post-operative wound care.2,4.
The most common antibiotics used in the management of CABG associated infections different antibiotics been investigated. Different generation of Cephalospurins are most important study groups of antibiotics. Second and third generations are regarded to have higher advantages in comparison to the first generations. This comparison also indicated the superiority of this group of antibiotics in comparison to Penicillins and or Glycopeptides without gram negative cover. However this advantage is not related to reduction in deep sternal wound infection or other types of SSIs, but instead, it is related to a noticeable reduction in post-operative pneumonia and overall mortality. It is important to appreciate that this reduction is not refer as a primary goal for surgical prophylaxis. 2,3
Methods
To compare the antibiotics prophylaxis utilization in ShahidRajaei Heart Center, Tehran, Iran, with international recognized standards, we conducted an observational 6-month study at this center, by a sectional, descriptive and prospective study. The choice, dose, duration and frequency of post-operational antibiotics prophylaxis used on patients undergoing CABG surgery at abovementioned center. This review including the antibiotics administrated to the patients during their hospitalization in ICU as well as CCU in ShahidRajaei hospital. The trials were done on 200 patients with during the period beginning on 30 September, 2012 up to 30 April 2013. The concentration is mostly on utilization of antibiotics of Cephalosporin (Ceftriaxone and Cefazolin), Vancomycin, and ciprofloxacin as the most used ones in this medical center. The data gathered was done on the bases of a questionnaire including the patients’ history and the administrated antibiotics. The main demographic parameters recorded were age, sex, marital status, BMI, diagnosis, type of surgery (either CABG or a combined one), main disease and comorbidities, drug allergies, habits, number of hospitalization, drugs administrated in ICU and CCU, dose and frequencies, duration, drug interactions, cost and other laboratory parameters, and finally post-op infection and secondary infections. These data were collected through patient disease history, physician’s order (patient’s record), as well as verbal inquiry from direct physician or nurses. Each case is independently compared with the respective guideline in order to study its consistency (in dose, duration and frequency) to globally-applied guideline, i.e. ASHP, ACC/AHA, NHS ESC etc. The collected data have been analyzed by SPSS software, in order to figure out the descriptive report. A flowchart diagram describing the methodology undertaken, is shown in Figure 1 below.
For the statistical analysis, data were described as mean ± standard deviation (SD) or median (Inter Quartile Renge) for interval and count (%) for categorical variables.We was showed the fitness of interval variables with one-sample Kolmogorov-Smirnov testComparison between subgroups was performed by chi-square test for categorical variables and Mann-Whitney U test for numerical variablesP value < 0.05 considered as statistically significant result. SPSS 15 for Windows (SPSS Inc. Chicago, Illinois) was used for statistical analysis.
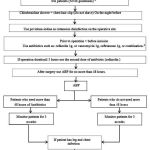 |
Figure 1: Trial flow diagram.
|
Results
As the background characteristics, 130 patients (37 women, 93 men) with mean age of 64±8 years participated. We also took previous medical conditions or comorbidities into considerations 78.5% of dose patient are already diagnose with CAD as a top known hospitalization background. Other Background and demographic descriptive data are presented in table 1.
Table 1: Back ground and demographic descriptive data (n = 180)
| Characteristic/ Variable | Mean ± SD/ count (%) |
| Age (year) | 64±8 |
| >65 | 55(42.3) |
| Gender (F/M) | 37/93 |
| Body Mass Index | |
| <30 | 104(80) |
| >30 | 26(20) |
| Marital Status | |
| Single | 10(7.7) |
| married | 120(92.3) |
| hospitalization background | 74(56.9) |
| Diagnosis (CAD) | 102(78.5) |
| CABG | 84(64.6) |
| Main disease (CAD) | 95(73.1) |
| Comorbidity | 97(74.6) |
| Diabetes | 49(37.7) |
| Auto-immune disease | 3(2.3) |
| Heart disease | 68(52.3) |
| Liver disease | 10(7.7) |
| Kidney disease | 13(10) |
| Drug allergy | 4(3.1) |
| Habits | 30(23.1) |
| Dialysis | 5(3.8) |
| Hospitalization during the recent 30 days | 29(22.3) |
Most of the Antibiotics prescriptions did not follow the guidelines in proper manner. However Vancomycin prescriptions had the most compliance with the guideline in term of correct doses being administrated. However the duration of use for this antibiotic did not follow the guideline. comparison with the guideline are presented in table 2.
Table 2. Frequency of antibiotic prescriptions followed the guideline c.
| Compliance with the guideline (%)b | ||||
| Variable | count (%)a | (reception intervals) | ||
| (dose) | (duration) | |||
| Ceftriaxone (IV*) | 74(56.9) | 0 | 0 | 0 |
| Vancomycin(IV) | 99(76.2) | 97(97.9) | 7(0.1) | 97(97.9) |
| Cefazolin (IV) | 50(38.5) | 50(100) | 4(0.1) | 50(100) |
| Ciprofloxacin (IV) | 18(13.8) | 17(94.4) | 0 | 18(100) |
| Ampibactam (IV) | 1(0.8) | 0 | 0 | 0 |
| Imipenem (IV) | 2(1.5) | 0 | 0 | 0 |
| Meropenem (IV) | 6(4.6) | 0 | 0 | 0 |
| Cefepim (IV) | 3(2.3) | 0 | 0 | 1(33.3) |
| Clindamycin (IV) | 2(1.5) | 2(100) | 0 | 2(100) |
| Tazocin (IV) | 3(2.3) | 0 | 0 | 0 |
| Amikacin (IV) | 1(0.8) | 0 | 0 | 0 |
| Gentamycin(IV) | 1(0.8) | 1(100) | 0 | 1(100) |
| Targocid (IV) | 3(2.3) | 0 | 0 | 0 |
| Metronidazol (PO**) | 1(100) | 0 | 0 | 1(100) |
| Cotrimoxazole (IV) | 1(100) | 0 | 0 | 0 |
| Colistin (IV) | 1(100) | 0 | 0 | 0 |
a = percentage computed base on all the patients.
b = percentage computed base on antibiotic prescription not all thepatients. c= ASHP Guideline
IV stands for Intravenous
PO stands for Per Oral
Significant interactions were observed between antibiotics and Anticoagulants, ACEIs, Antiarrhythmic drugs, Cardiac Glycosides, Insulin, Minerals, Vitamins, Steroids, Laxatives, Mucolytic and Anti hyperkalemia. Detailed Interaction, between ICU medication and antibiotics are listed in Table 3.
Table 3: Interaction between ICU medication and antibiotics
| Drug Interaction | |||
| Characteristic/ Variable | P value | ||
| Yes | No | ||
| N=56 | N=74 | ||
| PPIs (n=128) | 55(98.2) | 73(98.6) | 0.842 |
| Analgesics (26) | 10(17.9) | 16(21.6) | 0.595 |
| Antiplatelets (n=126) | 54(96.4) | 72(97.3) | 0.776 |
| Anticoagulants (n=45) | 43(76.8) | 2(2.7) | <0.001 |
| Antilipid drugs(Statins) (n=120) | 51(91.1) | 69(93.2) | 0.645 |
| B blockers (n=122) | 50(89.3) | 72(97.3) | 0.060 |
| ACEIs (n=89) | 32(57.1) | 57(77) | 0.016 |
| CCBs (n=10) | 6(10.7) | 4(5.4) | 0.261 |
| Nitrates (n=11) | 7(12.5) | 4(5.4) | 0.150 |
| Duretics (n=45) | 24(42.9) | 21(28.4) | 0.086 |
| ARBs (n=29) | 15(26.8) | 14(18.9) | 0.286 |
| Antiarrhythmic drugs (n=7) | 7(12.5) | 0 | 0.002 |
| Heart Glycosides (n=3) | 3(5.4) | 0 | 0.044 |
| Insulin (n=8) | 6(10.7) | 2(2.7) | 0.060 |
| Antifungal drugs (n=4) | 3(5.4) | 1(1.4) | 0.190 |
| Antipsychotic drugs (n=4) | 2(3.6) | 2(2.7) | 0.787 |
| Antianxiety drugs (n=8) | 5(8.9) | 3(4.1) | 0.252 |
| Antidepressant drugs (n=3) | 1(1.8) | 2(2.7) | 0.730 |
| Minerals (n=26) | 18(32.1) | 8 (10.8) | 0.003 |
| Vitamins (n=27) | 17(30.4) | 10(13.5) | 0.019 |
| Synthetic erythropoietin (n=2) | 1(1.8) | 1 (1.4) | 0.842 |
| Steroids(n=4) | 4(7.1) | 0 | 0.020 |
| Laxatives (n=6) | 5(8.9) | 1 (1.4) | 0.041 |
| Respiratory drugs (n=14) | 7(12.5) | 7(9.5) | 0.580 |
| Mucolytic (n=16) | 11 (19.6) | 5(6.8) | 0.027 |
| Estrogens (n=1) | 1(1.8) | 0 | 0.249 |
| Thyroid drugs (n=1) | 1(1.8) | 0 | 0.249 |
| Serum Amino plasma & Intra lipid (n=2) | 1(1.8) | 1(1.4) | 0.842 |
| Anti hyperkalemia (n=3) | 1(5.4) | 0 | 0.044 |
| Anti gout (n=1) | 1(1.8) | 0 | 0.249 |
| Anti hypertensive drugs(vasodilators) (n=2) | 2(3.6) | 0 | 0.101 |
| Gastro entricdrugs (n=1) | 1(1.8) | 0 | 0.249 |
We observed that median cost in Vancomycin, Cefazolin and Ciprofloxacin groups were significantly greater than those who did use other antibiotics. Median costs of more frequent antibiotic prescriptions shown in table 4.
Table 4. Relationship between costa and frequency of antibiotic prescribings
| Antibiotic prescription | |||||
| Characteristic/ Variable | P value | ||||
| No | Yes | ||||
| Ceftriaxone (IV) | 498000(198000-655500) | 417600(417600-483600) | 0.830 | ||
| Vancomycin (IV) | 198000(198000-500000) | 417600(417600-556800) | <0.001 | ||
| Cefazolin (IV) | 417600(417600-960000) | 422250(198000-498000) | 0.002 | ||
| Ciprofloxacin (IV) | 417600(417600-498000) | 2038400(1099200-8126800) | <0.001 | ||
| a = Iranian Rials | |||||
The median cost was significantly lower in patients who did not follow the guidelines compared with those who followed the guidelines. This could be due to the fact that guidelines are recommending more expensive antibiotics.
Table 5: Comparison of cost between patients in whom percentage dose of antibiotics followed the guideline and in whom did not.
| Compliance of dose with the guideline | ||||
| Characteristic/ Variable | P value | |||
| No | Yes | |||
| Ceftriaxone (IV) (n=74) | 417600(417600-539700) | – | – | |
| Vancomycin (IV) (n=99) | 198000(198000-330000) | 417600(417600-600000) | <0.001 | |
| Cefazolin (IV) (n=50) | 417600(417600-960000) | 422250(198000-498000) | 0.002 | |
| Ciprofloxacin (IV) (n=18) | 417600(417600-498000) | 2156800(1178600- | <0.001 | |
| 8725200) | ||||
Table 6.:Duration of Antibiotics utilization after surgery
| Duration (hours) | |||
| Characteristic/ Variable | |||
| <48 | 48-92 | >92 | |
| AmpCefteriaxone (n=74) | 6(8.1) | 62(83.8) | 6(8.1) |
| AmpVancomycin (n=99) | 9(9.1) | 79(79.8) | 11(11.1) |
| AmpCefazolin (n=50) | 4(8) | 39(78) | 7(14) |
| AmpCiprofloxacin (n=18) | 0 | 8(44.4) | 10(55.6) |
| AmpAmpibactam (n=1) | 0 | 0 | 1(100) |
| AmpImipenem (n=2) | 1(50) | 1(50) | 0 |
| AmpMeropenem (n=6) | 1(16.7) | 0 | 5(83.3) |
| AmpCefepim (n=3) | 1(33.3) | 0 | 2(66.7) |
| AmpClindamycin (n=2) | 0 | 0 | 2 (100) |
| AmpTazocin (n=3) | 0 | 0 | 3 (100) |
| AmpAmikacin (n=1) | 0 | 0 | 1(100) |
| AmpGentamycin (n=1) | 0 | 1(100) | 0 |
| AmpTargocid (n=3) | 1(33.3) | 0 | 2(66.7) |
| TabMetronidazol (n=1) | 1(100) | 0 | 0 |
| AmpCotrimoxazole (n=1) | 0 | 1(100) | 0 |
| AmpColistin (n=1) | 0 | 0 | 1(100) |
Discussion
Since prior to the present study actually no exact image and approved data were available about Antibiotics prophylaxis in patients undergoing CABG, this study is intended to set a cornerstone for further investigations concerning Antibiotics prophylaxis for CABG patients, by reviewing the existing data and experiments in ShahidRajaei Heart Center, as a tertiary heart care center in Tehran, Iran. Antimicrobial prophylaxis is used as an essential supplementary to techniques used for reducing the probability of SSIs after different types of surgeries, particularly cardiovascular surgery. Nevertheless, the consent of essentiality of utilization of Antibiotics is not projected to the methodology of this utilization, and the compliance of Antibiotics Prophylaxis to internationally recognized guidelines is perennially remaining as a controversial issue. 7,12,13 This study is done by assessment of Antibiotics prescribed to the patients undergoing CABG operation in a 9-month interval in Shahid Rajaei Heart Center.
Compliance to Guidelines
After gathering and analyzing the data as described above, we evaluated compliance of the obtained data to either of internationally-recognized guidelines, such as American Society of Health System Pharmacists (ASHP)8, Society for Healthcare Epidemiology of America (SHEA)9, National Health Service (NHS)10, American College of Cardiology foundation /American Heart Association (ACC/AHA)27, European Society of Cardiology (ESC) and Centers for Disease Control and prevention (CDC)30. SHEA advices replacing of Cefazolin by Vancomycin and Rifampicin for coronary artery bypass, and remains almost silent about the dose and during of its utilization. Therefore, this guideline shows little relevancy to our factual studies in this article. The NHS guideline, having strongly recommended antibiotics prophylaxis for a duration of not more than 48 hours, and the names and regimen of antibiotics utilization is not described in this guideline in details. It was observed the drug utilization in ShahidRajaei Heart Center shows its most compliance with ASHP guideline.8 In the latter guideline, Cefazolin, cefuroxime and Cefamendole are recommended as the main agents, while Clindamycin, Vancomycin and Azteroname are recommended as the alternative agents in patients with β- Lactam Allergy.8 Concerning the duration, this guideline has recommended a antibiotics prophylaxis duration of 24 up to 48 for post-CABG patients, except for the ones suffering drain, catheter, MRSA or SSI complications, for whom a utilization of more than 48 hours are recommended, according to the case in question.
Considering the ASHP as the referent guideline, along with other guidelines (i.e. CDC,NHSetc), the following results have been observed: I. Ceftriaxone, as the second mostly common utilized Antibiotics in this center, is not recommended in ASHP and other afore-named guidelines.
Concerning other three prevalent Antibiotics, i.e. Vancomycin, Cefazolin and Ciprofloxacin, an utter compliance to the dose and intervals recommended by the guidelines is observed (98% to 100%).
From the other side, an utter non-compliance in duration of utilization is observed (99.9% to 100%). These non-compliances are mostly represented as prolonged duration of utilization (highly longer than is recommended in the guidelines).
Some Antibiotics recommended by guidelines, such as Gentamycin and Clindamycin are scarcely utilized in prescribed regimen, giving their place to Ceftriaxone.
For further description, refer to table No. 2. For a detailed description of Antibiotics utilization duration distribution refer to Table No. 6, above.
The prevalence of prescribed Antibiotics
The most prevalent Antibiotics utilized is this center for reduction of SSI after CABG, are Vancomycin, Ceftriaxone, Cefazolin and Ciprofloxacin. Other utilized Antibiotics are mentioned in Table No. 2, above.
Drug Interactions
Drugs interactions are mostly observed among the drugs prescribed during patients’ stay in ICU.
According to bnf standard handbook 11, and as tabulated in Table No. 3 above, Anticoagulants and Minerals show a significant drug interaction P Values.
Cost Evaluation
Cost impacts of ABP is one of the factors involved in selection of antibiotics. As instance in the study by Stephan Harbarthet al.7 ceftriaxone is rolled out of the antibiotic regimen, in spite of being potentially suitable in prolonged surgeons14, because of its cost implications.7 As seen in Table No. 4 above, among the most prevalent Antibiotics, the P Value correspondent
to Ceftriaxone prescription implies no significant relationship between cost and prescription, while for other three antibiotics (Vancomycin, Cefazolin and Ciprofloxacin) we can observe a significant relationship. This relationship can be expressed in this way that for the latter three Antibiotics, the cost significantly increases in the case of prescription. This observation is endorsed by Table No. 5 which concern a comparison between these Antibiotics based on their guideline.
Although the principles of surgical Antibiotic prophylaxis have been outlined on numerous occasions, misuse of antibiotics for this purpose (especially prolonged utilization) is always a controversial issue.16,17,18,28,29 The situation is even worse for Iranian applications. In a pre-operation AB prophylaxis prescription study performed in Shariati Hospital, Tehran, Iran (S. Afhami et al. 2011), a compliance of 4.6 percent to the guidelines was reported.19 Although it’s demonstrated that prolonging ABP beyond 48 hours after CABG surgery is still frequently practiced in spite of globally recognized standard recommendations, but does not show any significant positive effect on reducing the risk of SSI.3,7 Moreover, it results in an increased risk of acquired antibiotic resistance and should therefore be avoided.7 Some researchers have suggested that extended prophylactic use of antibiotics is appropriate when drains or catheters remain in situ for several days after cardiac surgery.7 though according to ASHP guideline there is no data to support the continuation of antimicrobial prophylaxis indwelling drains and intravascular catheters.1,15,20-24
In a study performed by Inkster T, a shift away from traditional Cephalosporins is widely observed, so that a combination of Fluxacilin and gentamycin is reported as the most common regimen.5 The pre-operation Antibiotics Prophylaxis study by S. Afhami et al.19 demonstrates a result of 40.7% and 4.6% of compliance of type and duration of the prescribed Antibiotics with guideline, respectively; while these ratios in our study (for Vancomycin as the most prevalently used Antibiotic) is demonstrated as 76.2% and 0.1% respectively. Other results from similar studies performed in different countries around the world such as systematic review published by Mertz et al25, demonstrate though the type and dose of selection of Antibiotics are usually done according to applicable guidelines, use of prophylactic regimen in cardiac surgery is evidently prolonged. Investigation by suggests that post-operation Antibiotics Prophylaxis for a duration of 24 hours along with utilization of β-lactams, only prevents post-operation pneumonia, but does not significantly show prevention from DSWI and SSIs.4,10,15,26 On the other side, the duration of more than 48-hours does not show any significant positive effect. A study performed in Harvard Medical University7 supports that ABP is sufficient to be utilized up to 48 hours after CABG surgery, and prolonged utilization further to 48 hours not only shows no improvement in reducing the SSI, but also results in antimicrobial resistance.1,27
Conclusion
As shown above, the most prominent problem in Antibiotics prophylaxis utilization in the target medical center is the non-compliance of selection and duration of antibiotics to the applicable guidelines. This problem can be illustrated to be mainly resulted from some reasons such as lack of adequate trainings to the nurses in order to keep them updated about the most recent finding and optimum methods, lack of evaluation forms and procedure in order to register and evaluate he utilized Antibiotics, non-availability of treatment protocols in medical centers, inadequacy of team working mentality, disregarding the cost of antibiotics regimen and hoteling, and lack of e-archive in the target medical center.
References
- Fred H. Edwards, MD, Richard M. Engelman, MD, Peter Houck, MD, MPH, et al. The society of thoracic surgeons practice guideline series: Antibiotic prophylaxis in cardiac surgery, part I: Duration, Ann ThoracSurg 2006; 81:397-404.
- Ruth Kappeler, Margaret Gilham and Nicholas M. Brown. Antibiotic prophylaxis for cardiac surgery. J AntimicrobChemother 2012; 67: 521- 522.
- Lador A, Nasir H, Mansur N et al. Antibitic prophylaxis in cardiac surgery: systemaic review and meta-analysis. J AntimicrobChemother 2012; 67: 541-50.
- National Institute for Health and Clinical Excellence. Surgical Site Infection CG74. London: National Institute for Health and Clinical Excellence, 2008.
- Inkster T. Antibiotic prophylaxis for cardiac surgery: a shift away from traditional Cephalosporins ? J CardiothoracVascAnesth 2009; 23: 933-5.
- Alicia J. Mangram, MD; Teresa C. Horan, MPH, CIC; Michele L. Pearson, MD, et al. Guideline for prevention of surgical site infection,1999. Vol 20, No.4.
- Stephan Harbarth, Matthew H. Samore, Debi Litchenberg and Yehuda Carmeli. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation. 2000; 101:2916-2921.
- Dale w. Bratzler, E. Patchen Dellinger, Keith M. Olsen et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health-Syst pharm. 2013; 70:195-283.
- Denis Spelman , FRACP; Glenys Herrington et al. Clinical, microbiological, and economic benefit of a change in antibiotic prophylaxis for cardiac surgery. Source: Infection Control and Hospital Epidemiology, Vol. 23, No. 7 (July 2002) , pp. 402-404.
- Scottish Intercollegiate Guidelines Network. Antibiotic prophylaxis in surgery: A Naional Clinical Guideline. 2008.
- BNF 59, BMJ Group and Pharmaceutical Press 2010, ISBN: 9780 85369 9293.
- Kreisel D, Savel TG, SilverAL, et al. Surgical antibiotic prophylaxis and Clostridium difficile toxin positivity. Arch Surg. 1995;130:989-993.
- Classen DC, Evans RS, pestotnik SL, et al. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992;326:281-286
- Boxma H, Broekhuizen T, Patka P, et al. Randomised controlled trial of single-dose antibiotic prophylaxis in surgical treatment of closed fractures: the Dutch Trauma Trial. Lancet. 1996;347:1133-1137.
- Bratzler D, Houck P. Antimicrobial prophylaxis for surgery: an advisory statement from the national surgical infection prevention project. Clin Infect Dis 2004; 38: 1706 – 15.
- Alexiou VG, Ierodiakonou V, Peppas G et al. Antimicrobial prophylaxis in surgery: an international survey. Surg Infect (Larchmt) 2010; 11: 343 – 8.
- Haydon TP, Presneill JJ, Robertson MS. Antibiotic prophylaxis for cardiac surgery in Australia. Med J Aust 2010; 192: 141 – 3.
- Al-Momany NH, Al-Bakri AG, Makahleh ZM et al. Adherence to international antimicrobial prophylaxis guidelines in cardiac surgery: a Jordanian study demonstrates need for quality improvement. J Manag Care Pharm 2009; 15: 262 – 71.
- Afhami Sh. MD , EsmailpourBazaz N. MD , BoujarArani N. MD , Sayadi L. Antibiotic prophylaxis before surgeries. Iranian Operation Journal , Number 3 , 1390.
- Alphonso N, Anagnostopoulos PV, Scarpace S et al. Perioperative antibiotic prophylaxis in pediatric cardiac surgery. Cardiol Young. 2007; 17:12-25.
- Nateghian A, Taylor G, Robinson JL. Risk factors for surgical site infections following open-heart surgery in a Canadian pediatric population. Am J Infect Control. 2004; 32:397- 401
- Bratzler DW, Houck PM, Richards C et al. Use of antimicrobial prophylaxis for major surgery: baseline results from the National Surgical Infection Prevention Project. Arch Surg. 2005;140:174-82.
- Lee KR, Ring JC, Leggiadro RJ. Prophylactic antibiotic use in pediatric cardiovascular surgery: a surgery of current practice. Pediatr Infect Dis J. 1995; 14:267-9.
- McCarthy PJ, Patil S, Conrad SA et al. International and specialty trends in the use of prophylactic antibiotics to prevent infectious omplicationsafter insertion of external ventricular drainage devices. Neurocrit Care. 2010; 12:220-4.
- Mertz D, Johnstone J, Loeb M. Does duration of perioperative antibiotic prophylaxis matter in cardiac surgery? A systematic review and meta-analysis. Ann Surg 2011; 254: 48 – 54.
- Mangram AJ, Horan TC, Pearson ML et al. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control HospEpidemiol 1999; 20: 250 – 78; quiz 279 – 80.
- Eagle KA, Guyton RA, Davidoff R et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery). J Am CollCardiol 2004; 44: e213 – 310.
- Prado MA, Lima MP, Gomes Ida R, Bergsten- Mendes G. The implementation of a surgical antibiotic prophylaxis program: the pivotal contribution of the hospital pharmacy. Am J Infect Control 2002; 30(1): 49-56.
- García- Vázquez E, FernándezLobato B, Pareja A, Gómez J, de la Rubia A. Pharmacoeconomic results of introducing antimicrobial prophylaxis in surgery at a university hospital. Cir Esp. 2008; 84(6): 333-6.
- Kanayama M, Hashimoto T, Shigenobu K, Oha F, TogawaD.Effective prevention of surgical site infection using a Centers for Disease Control and Prevention guideline-based antimicrobial prophylaxis in lumbar spine surgery. J Neurosurg Spine 2007; 6(4): 327-9







