Manuscript accepted on :March 15, 2016
Published online on: 21-04-2016
Plagiarism Check: Yes
Mahtab Alimardan1,2 ,Parisa Ziarati *1,3 , Rana Jafari Moghadam1,4
1Young Researchers and Elite Club, Pharmaceutical Sciences Branch, Islamic Azad University, (IAUPS), Tehran, Iran 2Department of Food Sciences and Technology , Faculty of Advanced Sciences and Technology , Pharmaceutical Sciences Branch, Islamic Azad University ,Tehran -Iran (IAUPS ) 3 Department of Medicinal Chemistry, Faculty of Pharmacy ,Pharmaceutical Sciences Branch, Islamic Azad University, Tehran-Iran ( IAUPS) 4Pharmacy Faculty, Pharmaceutical Sciences Branch ,Islamic Azad University , Tehran-Iran (IAUPS ) Corresponding Author Email : ziarati.p@iaups.ac.ir
DOI : https://dx.doi.org/10.13005/bpj/924
Abstract
Barberry is well known and naturally adapted in Iran, and it has been extensively used as a medicinal plant in traditional medicine. The fruit of the barberry is used in medicine to treat liver, neck and stomach cancer, for purification of the blood and for breath freshness. Iran is the main center for distribution of genus Barberry vulgaris and Barberry integerrima and one of the richest countries in the world as regards genetic resources of medicinal and wild unknown plants that some of them are export . We have studied on potential ability of Barberry integerrima residue potential as an adsorbent of heavy metal . B. integerrima barberry fruits were collected in 2015 from Qaen in south Khorasan province in the north-east of Iran and put in contaminated soil samples by heavy metals. Aerial parts of growing coriander in every ten days in companion of B. integerrima fruits residue in the soils were separated in 30 days and digested by wet method according the standard protocol for measuring Cadmium ,Chrome (III) and (VI) , Nickel and Lead. Mean values were calculated, and analysis of variance (ANOVA) and Student’s t-test were performed. The results of present study revealed adsorption capacity of Cr (VI), Cr(III), Pb and Cd by barberry residue was investigated in a batch system by considering the effects of various parameters like contact time, initial concentrations, pH , temperature, absorbent dose. The results of this study revealed that B. integerrima fruit barberry residue can accumulate high level of lead, Cadmium and Chrome (VI) and (III) in a short time and their uptake rate by vegetable and edible plant is significantly affected by their concentrations in the contaminated soil (p<0.05). A contact time of 10 days by B. integerrima was found to be optimum and 89.2% Cr (VI), 78.9% Cr(III), 95.6% Pb and 43.6% Ni was remained in soil while a few amounts of these heavy metals being uptake by edible vegetable – coriander . Experimental results showed that low cost bio-sorbent was effective for the removal of pollutants from soil.
Keywords
Barberry integerrima; adsorption; Iran; Coriander; Contaminated Soil
Download this article as:| Copy the following to cite this article: Alimardan M, Ziarati P, Moghadam R. J. Adsorption of Heavy Metal Ions from Contaminated Soil by B. integerrima Barberry. Biomed Pharmacol J 2016;9(1) |
| Copy the following to cite this URL: Alimardan M, Ziarati P, Moghadam R. J. Adsorption of Heavy Metal Ions from Contaminated Soil by B. integerrima Barberry. Biomed Pharmacol J 2016;9(1). Available from: http://biomedpharmajournal.org/?p=6790 |
Introduction
Barberry family includes about 650 species of plants and shrubs which are often spiny. Root, stem, leaves and fruits are used in medicine and food industry. Zakaria Razi introduced two species of barberry in his book; black barberry that grows in mountains with stronger medicinal properties and red one that grows in valleys. In Iran two important barberry species are B. Integerrima (abi) and B. Vulgaris (poloei). B. Integerrima is a thorny shrub with fragile branches to a height of 1 to 3 meters [1].
Barberry is an Iranian exclusive plant which is generally cultivated around Birjand, South Khorasan, Qaen, Tabas, Gonabad and Kashmar. The Berberis vulgaris fruit is very useful as tonic for liver and heart; it prevents chronic bleeding, reduces mucus, purifies blood, and also reduces triglycerides, cholesterol and blood pressure. In addition it is effective in treatment of gall bladder, bleeding hemorrhoids, antiparasitic liver, diabetes, gout, kidney stones, colon cancer, prostate inflammation, malaria, fever, asthma[2] and neurological diseases [3].
B. Integerrima fruits are used to prepare juices. The use of barberry fruit as a natural food colorant rich in anthocyanins instead of harmful artificial ones was studied by researchers [4]. In natural fiber products barberry fruit’s extract used as a colorant shows a mild purple color [5]. Barberry bioactive compounds are widely used in medical and food industry [6]. Berberis integrrima (Syn: Berberis densiflora Boiss.&Buhse) is a medicinal shrub with yellow wood and obovate leaves, bearing pendulous yellow flowers succeeded by oblong red fruits. This plant belongs to the Berberidaceae and found in most regions of Iran, especially in northern and northeastern[7] .Due to having secondary metabolites such as Berberine, Oxyacanthine, Bermamine, Palmatine, Jateorrhizine, Columbamineand Berberubine, this plant has too much medicinal properties [7-9]. Barberis contain citric acid and malic acid and due to these possesses astringent, and anti scorbutic properties useful in inflammatory fevers, especially typhus, and scurvy, and in the form of a jelly, are very refreshing for irritable sore throat; syrup of berberis made with water is used as an excellent astringent gargle. The fresh juice of the fruit is also said to strengthen the gums and relieve pyorrhea when brushed on or applied directly to the gums. A decoction of the bark or berries has been found of service as a wash in aphthous sore mouth, and in chronic ophthalmia [10]. Various properties are listed for different parts of barberry plant and these properties have been confirmed in various research[11,13]. In addition to the antioxidant properties of Barberry fruit [11], a variety of alkaloids obtained from root and stem bark, which most important of them is Berberine [12]. Based on studies on barberry root extract and its main alkaloid (Berberine) these following properties are listed: Antioxidants [11],anti-inflammatory effects [12], hypoglycemia [13], hypolipidemic [14] ,collecting free radicals and finally reduction of oxidative stress [15]. However most of these studies performed in animal models and used of berberine (root and stem extract of barberry). Given that barberry fruits contain polyphenols, pectin and gum, vitamin C and malic acid [16], the present study was designed to evaluate the effects of Berberis integerrima Bge. fruit aqueous extract on adsorbing heavy metals in contaminated soil and rescue soil for the optimum condition for growing edible vegetables and crops . Iran is the main center for distribution of genus Barberry vulgaris and Barberry integerrima . As Iran is one of the richest countries in the world as regards genetic resources of medicinal and wild unknown plants that some of them are export [17]. We have studied on potential ability of Barberry integerrima specie as its endemic specie found in some parts of Iran especially in in south Khorasan province in the north-east of Iran and its’ residue potential as an adsorbent of heavy metals in order to find an inexpensive adsorbent for the removal of Pb, Ni, Cd and Cr from agricultural soils.
Current research conducts adsorption of heavy metals by agricultural waste and by-product and follow low-cost environmentally friendly method for removal of heavy metals from contaminated soil.
Materials and Methods
integerrima (10 Kg) barberry fruits (ripped completely) were purchased in October 2015 from 5 gardens of Gaen in south Khorasan province in the north-east of Iran( figure 1) . To prepare the material barberry fruits were carefully purged of any branches, thorns, leaves, stones and other waste substances. Then cleaned fruits were packed in plastic bags and were kept at -18°C freezer Equipment. To prevent discoloration and reduce effects of drying process fruits were dried in the oven at 50°C for 48 hours for B. integerrima fruits according to the amounts of moisture. Dried fruits were ground by Moulinex grinder. For 500 gram of barberry , 4liters of deionized water were added. Allowed the mixture to be soaked for about 12 hours and then boiled it for 3hours, first at 100°C for 1 hour and then heating up to 80°C . After cooling the heated mixture its’ precipitations in the extracted fruit juice of B. integerrima was filtered and separated from colorful solution by Quantitative Ash less Filter Paper: Whatman – Grade 41.The fruit residue were added to the studied composite soils in 30 Coriander cultivated vases .
Soil Sampling
A composite soil sample was collected from depth of 0-35 cm from a yard in the center of Tehran in order to simulate the conditions of soils in the contaminated lands with industrial sewages. 30, 15, 30, 25, 20, 15, 5, 20, 20, 30 and 30 mM/L of Pb(NO3)2, Cd (NO3)2, KNO3, Zn NO3 , MnSO 4 , CrCl3,K2 Cr2 O7 , K2 SO 4 . 5H 2 O, CaHPO4 and Ni SO4 respectively and 200 g of residue of B. integerrima barberry fruits and separating (ratio 10 :1) were added. Composite soil samples (depth of 0-35 cm) were collected of Heavy metal contaminated soil by coriander cultivated grown and not grown in it by different pHs after every 10 days in 30 day studying. Metal contents were detected by Atomic Absorption Spectrophotometer by wet digestion method in Research Laboratory in Pharmaceutical Sciences Branch University.
 |
Figure 1: The location of collecting B. integerrima barberry |
At the beginning of study, soil profile characteristics were observed and recorded by a packet penetrometer (Cl-700A, soil Test Inc., USA). Soil samples were mixed, homogenized and separated into three parts, 1/3 of each samples was air-dried and pass through a 2 mm sieve in order to determine p and k content, pH and electrical conductivity and particle-size distribution. The other 2/3 was passed through a 2 mm sieve without drying and 1/3 of it used to determine heavy metals concentration by Atomic Absorption Spectroscopy (AAS) after digestion with aqua-regia. The samples were analyzed by an Atomic Absorption Spectrophotometer Model AA-6200 (Shimadzu, Japan) using an air-acetylene flame for heavy metals: Chrome, Nickel, Lead and Cadmium, using at least five standard solutions for each metal. All necessary precautions were taken to avoid any possible contamination of the sample as per the AOAC guidelines [18-20].
Sampling method
Aerial parts of coriander in every ten days in companion of B. integerrima fruits residue were separated in 30 days and washed and digested by wet method according the standard protocol for measuring Cadmium ,Chrome (III) and (VI) , Nickel and Lead. Bioaccumulation factors (BAF-s) were calculated for heavy metal content of plant parts (mg/kg) / heavy metal content of soil (mg/kg), for each metal [ 17].
All coriander samples were watered each day by tap water (Tehran tap water). The studied samples were managed by the same light situation and some circumstances in order to be compared with each other due to determine the ability of B. integerrima in adsorbing Lead, Cadmium and Nickel from soil and its potential to avoid transferring heavy metals to coriander and keep safe the eating vegetable .
Physical and chemical properties and concentrations of heavy metals (Cadmium, Nickel and Lead,) in soils, before and after adding B. integerrima fruit barberry residue in the growth period of cultivated coriander were measured in every ten days. In order to assess amount of heavy metals in the soil samples, heavy metal concentrations in soils of studied vases were determined by atomic absorption spectrophotometer [21-26].
Statistical analysis
The values reported here are means of five values. Data were tested at different significant levels using student t-test to measure the variations between the contaminations in soil and coriander parameters before and after treated by B. integerrima fruit residue . One way analysis of variance (One-ANOVA) was used for data analysis to measure the variations of metal concentrations using SPSS 22.0 software (SPSS Inc, IBM, Chicago, IL).
Results and Discussion
Chemical extraction of the soil profile before adding specified amounts of heavy metals is shown in the table 1. Data is averages of the profiles.
Table 1- chemical characteristics of the soil profile at the studied vases (before adding chemical substances and pre growing of studied Coriander in the presence of B. integerrima fruits residue
| Layer
(depth cm) |
pH (H2O) | Electrical conductivity
dS/cm 1:1 |
NO3-N
mg/kg DW |
NH4-N
mg/kg DW |
| 1 (0-10) | 6.4 | 0.38 | 62.1 | 8.92 |
| 2 (10-20) | 6.5 | 0.19 | 33.9 | 8.33 |
| 3(20-35) | 6.7 | 0.30 | 26.4 | 7.86 |
Plant availability of certain heavy metals depends on soil properties such as soil pH and contain exchange capacity and on the distribution of metals among several soil fractions. The fractionation of Pb, Cr, Ni, and Cd in coriander cultivated control soil and in soils treated by B. integerrima fruit barberry residue is completely determined due to find out the adsorption ability of heavy metals by barberry residue in contaminated soil samples.
Results showed B. integerrima fruit barberry residue adsorption for all heavy metals in treated soil were affected significantly by barberry residue and the residue not only affected contaminated soil and can adsorb lead, Cadmium , Chrome and Nickel after 10 days (p<0.001) more than other studied but also adding barberry residue have reduction and rescue effect in taking up heavy metals especially in bio-adsorbing Cadmium and Nickel more than other heavy metals studied and it keeps edible vegetable safe for eating . In figure 2 the treating contaminated soil trend by this residue fruit indicates that dried B. integerrima fruit parts in the soil which is enriched soil by mineral elements and vitamins, it can be consider as a suitable method for rescuing soil by its relatively large ratio of biomass concentration of the contaminant to the soil concentration.
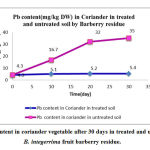 |
Figure 2: Lead content in coriander vegetable after 30 days in treated and untreated soil by B. integerrima fruit barberry residue. |
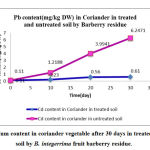 |
Figure 3: Cadmium content in coriander vegetable after 30 days in treated and untreated soil by B. integerrima fruit barberry residue. |
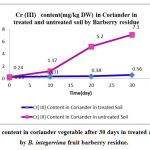 |
Figure 4 :Cr (III) content in coriander vegetable after 30 days in treated and untreated soil by B. integerrima fruit barberry residue. |
Chromium as a pure metal has no reported human or environmental toxicity effects. Both acute and chronic toxicity of chromium are mainly caused by hexavalent chromium compounds (Cr VI). Hexavalent chromium is considered the most hazardous of all forms, and in welding fume it is a suspected human carcinogen. DNA damage in welders has been associated with hexavalent chromium exposure [27] . This is consistent with the classification of hexavalent chromium as a human lung carcinogen [28-29]. The ratios of adsorbent/heavy metal contents were calculated to indicate the translocation efficiency of Pb and Cr from soil to the adsorbents while for the results of nickel translocation was not significant. The Cr (III) concentrations in samples after 10, 20, 30 days are higher in soil than Cr(VI), hence the translocation factor ratios were less than one for Cr (VI) treated samples shows that this method is highly suitable for adsorbing Cr(III) to the Cr (VI). Even though the Cr(VI), concentration of contaminated soil of Cr treated samples in 10 days treated samples is higher compared to controls solutions, the extent of metal accumulation and uptake efficiency of Cr(III) was lower than that of Cr(VI), in long term contaminated situation study. Most of coriander vegetable samples in Cr (VI) samples deformed after 30 days remaining in contaminated soils.
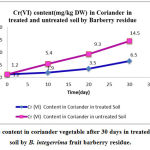 |
Figure 5 : Cr (VI) content in coriander vegetable after 30 days in treated and untreated soil by B. integerrima fruit barberry residue. |
The results of present study revealed adsorption capacity of Cr (VI), Cr(III), Pb and Cd by barberry residue was investigated in a batch system by considering the effects of various parameters like contact time, initial concentrations, pH , temperature, absorbent dose. The adsorption was time and metal dependent and the maximum adsorption was observed at lead contamination. Moreover, contact time of different heavy metals in the contaminated soil showed significant (p <0.05) and positive correlation with contents of Pb (r = +89 to r = +96), Cr6+ (r = +74 to r = +81), Cr 3+ (r = +79 to r = +83), Ni (r = +30 to r = +36) in the contaminated soil and B. integerrima fruit barberry residue respectively. The amounts of lead adsorbed increased significantly with increase contact time (p<0.005). The results of this study revealed that B. integerrima fruit barberry residue can accumulate high level of lead, Cadmium and Chrome (VI) and (III) in a short time and their uptake rate by vegetable and edible plant is significantly affected by their concentrations in the contaminated soil (p<0.05). A contact time of 10 days by B. integerrima was found to be optimum and 89.2% Cr (VI), 78.9% Cr(III), 95.6% Pb and 43.6% Ni was remained in soil while a few amounts of these heavy metals being uptake by edible vegetable – coriander . Experimental results showed that low cost bio-sorbent was effective for the removal of pollutants from soil.
The present investigation shows that the B. integerrima residue is effective and inexpensive adsorbent for the removal of Pb and Cr (VI) from soil.
This research conduct adsorption of heavy metals by agricultural waste and by-product and proved that this friendly method should gain more attention and research interest for the removal of heavy metals from contaminated soil due to its surface area, adsorption capacity and plenty abundant in nature must be followed seriously.
Also, this research suggests more investigations by other genera and families of cost-effective waste agricultural products applying as adsorbents for these heavy metals and other toxic metals such as cadmium, arsenic and Mercury.
Conflicts of Interest
None of the authors have any conflicts of interest associated with this study.
References
- Berenji Ardestani,S., Sahari,M.A., Barzegar, M., Abbasi, S. Some Physicochemical Properties of Iranian Native Barberry Fruits (abi and poloei): Berberis integerrima and Berberis vulgaris. J.Food Pharm.Sci. ,2013;1 :60-7.
- Pouyan, M. Barberry economical aspects and production, First Edition, Ghahestan Publishing, Birjand, Iran (In Farsi). 2008; pp 1-200.
- Fatehi, M., Saleh, T.M., FatehiHassanabad, Z., Farrokhfal, K.H., Jafarzadeh, M., Davodi, S.A. pharmacological study on Berberis vulgaris fruit extract. Journal of Enthopharmacology., 2005; 102: 46-52.
- Sharifi, A., Tavakolipour, H., Maskooki, A., Elhamirad, A. H. Evaluation of Barberry colour extraction. 18th National Congress on Food Technology, Mashhad, I. R. Iran (In Farsi). 2008; pp 268.
- Jimenez, C.D.C., Flores, C.S., He, J.,TAIAN, Q., Schwartz, S.J., Giusti, M.M. Characterization and preliminary bioactivity determination of Berberis boliviana Lecher fruit anthocyanins. Food Chemistry., 2011; 128 (3): 717-24.
- Fallahi, J., Rezvani Moghaddam, P., Nasiri Mahallati, M. Effects of harvesting time on quantitative and qualitative properties of B. vulgaris fruit. International Journal of Field Crops Research (In Farsi). 2010; 8 (2): 225-34.
- Ashraf, H., Heidari, R., Nejati, V. Antihyperglycemic and Antihyperlipidemic Effects of Fruit Aqueous Extract of Berberis integerrima Bge. in Streptozotocin-induced Diabetic Rats. Iranian Journal of Pharmaceutical Research ., 2014; 13 (4): 1313-18.
- Arayne, M.S., Sultana, N., Sher Bahadur, S. The berberis story: Berberis vulgaris in therzapeutics. J. Sci. ,2007;20: 83-92.
- Hosseinzadeh, H., Ramezani, M., Shafaei, H., Taghiabadi, E. Anticonvulsant effect of berberis integerrima root extract in Mice. J. Acupum. Meri. Stud. ,2013; 6: 12-7.
- Saeed Arayne , M., Sultana, N., Sher Bahadur, S. The Berberris Story: BERBERIS VULGARIS in THERAPEUTICS. Pak. J. Pharm. Sci., 2007; 20(1): 83-92.
- ZovkoKoncic ,M., Kremer, D., Karlovic, Kosalec, I. Evaluation of antioxidant activities and phenolic content of Berberis vulgaris L and Berberiscroatica Horvat. Food Chem. Toxicol. ,2010; 48: 2176-80.
- Mazumder, P.M., Das, S., Das, S., Das, M.K. Phytopharmacology of Berberis Aristata DC: a review. J. Drug Deliv. Therap. ,2011; 1: 46-50.
- Liu, L.Z., Cheung, S.C., Lan, L.L., Ho, S.K., Xu, H.X., Chan, J.C., Tong, P.C. Berberine modulates insulin signaling transduction in insulin-resistant cells. Mol. Cell Endocrinol., 2010;317: 148-53.
- Dong, S.F., Hong, Y., Liu, M., Hao, Y.Z., Yu, H.S., Liu, Y., Sun, J.N. Berberine attenuates cardiac dysfunction in hyperglycemic and hypercholesterolemic rats. Europ. J. Pharmacol. ,2011; 660: 368-74.
- Kumar, S., Kumar, D., Rakash, O. Evaluation of antioxidant potential, phenollic and flavonoid contents of hibiscus liliaceous flowers. EJAF Che. ,2008; 7: 2863-71.
- Pozniakovskii, V.M., Golub, O.V., Popova, D.G. The use of barberry berryes in Human Nutrition. Vopr. Pitan., 2003;78: 46-9.
- Ziarati, P., Kermanshah, A., Moslehishad, M. Adsorption Heavy Metal from Contaminated Water, by Modified Shell of wild Endemic almonds: Amygdalus Lycioided and Amygdalus Wendelboi. Bioscience & Biotechnology Research Asia., 2015; 12 (3): 2451-7.
- Ziarati, P., Khoshhal, Z., Asgarpanah, J., Qomi, M. Contaminations Of Heavy Metals In Tea Leaves, Finished Tea Products And Liqour In Gilan Province, Iran. International Journal of Farming and Allied Sciences. ,2013; 2 (13): 383-387.
- Mirmohammad-Makki , F., Ziarati ,P. Determination of Histamine and Heavy Metal Concentrations in Tomato pastes and Fresh Tamato (Solanum lycopersicum) in Iran. BBRA., 2014;11(2): 537-44.
- Yazdanparast ,S., Ziarati, P., Asgarpanah, J. Heavy Metals and Mineral Content and Nutritive Value of Some Iranian Manna. BBRA., 2014;11(2):1025-9.
- Becerra-Castro, C., Monterroso, C., Prieto-Fernández, A., Rodríguez-Lamas, L., LoureiroViñas, M., Acea, M.J., Kidd, P.S. Pseudometallophytes colonising Pb/Zn mine tailings: A description of the plant–microorganism-rhizosphere soil system and isolation of metal-tolerant bacteria. Journal of Hazardous Materials. 2012; 217(218 ) : 350-9.
- Damangir, A. A., Baghvand, A., Monavari, S. M., Moattar, F. Metal Pollution Assessment in Soil Samples of Mining Area, Shahr-E-Babak, Iran. International Journal of Advanced Biological and Biomedical Research. 2015; 3 (1):24-34.
- Ziarati, P., Nazif, M. H., Khandehrouy, M. . Decreasing Bio-toxicity of Fume Particles Produced in Welding Process by Aloe vera L. Oriental Journal of Chemistry. 2015; 31(4): (Spl Edn): 113-20.
- Ziarati,P., Ziarati,N.N., SYNERGIC PHYTOREMEDIATION POTENTIAL OF HEAVY METALS BY SORGHUM BICOLOR L. IN COMPANION OF SUNFLOWER (HELIANTHUS ANNUUS L.). International Journal of Pharmacy Practice and Pharmaceutical Sciences.2015, 2(4).
- Ziarati, P., Asgarpanah , J ., MirMohammad Makki, F. Phytoremediation of Heavy Metal Contaminated Water Using Potential Caspian Sea Wetland Plant: Nymphaeaceae. BIOSCIENCES BIOTECHNOLOGY RESEARCH ASIA, 2015; 12(3): 2467-73.
- Ziarati, P., Alimardan, M. Study on Increasing Efficiency of Phytoremediation in Cadmium and Nickel Contaminated Soil. Chemistry Journal .2015; 5(6): 86-92.
- Sellappa, S., Prathyumnan, S., Keyan, K.S., Joseph, S., Vasudevan, B.S., Sasikala, K. Asian Pac. J. Cancer Prev., 2010;11(1): 95–100.
- U.S. Department of Health and Human Services. Report on carcinogens. Twelfth edition, Public Health Service, National Toxicology Program; 2011.







