Manuscript accepted on :
Published online on: 06-01-2016
Plagiarism Check: Yes
Arif Yezdani1
Professor in Dept. of Orthodontics and Dentofacial Orthopaedics, Bharath University, Sree Balaji Dental College and Hospital, Narayanapuram, Pallikaranai,Chennai-600100
DOI : https://dx.doi.org/10.13005/bpj/711
Abstract
The purpose of this longitudinal study was to investigate whether acid phosphatase level changes in Gingival Crevicular Fluid (GCF) could be used to monitor bone turnover changes during human orthodontic tooth movement. Seven patients (2 males and 5 females; mean age, 23 years) were selected. Pre-Adjusted Edgewise Appliance (MBT 0.022 X 0.028-inch slot) was strapped up and aligning and leveling was completed prior to distalization of maxillary canines. The contralateral canine (CC) was not subjected to distal force and was used as the control tooth whereas the maxillary canine on the right side was used as the experimental tooth and was considered to be the distalized canine (DC). From the mesial and distal tooth sites of the DC and the CC, one µL of GCF was harvested with a (HirschmannR microcapillary pipette, Sigma AldrichR), before appliance activation, 1 hour after, and every week thereafter for a period of 28 days. The results were expressed as total ACP activity (U/L) determined spectrophotometrically at 300 C at 405 nm. One way Analysis of Variance (ANOVA) and Independent Samples t-test and Mann-Whitney U-test was done for comparison of enzyme activity among the pre-determined intervals and the SPSS computer program version 21 was used to carry out the statistical evaluation. The ACP activity in GCF was elevated in DC as compared with CC in the first week (3.964+0.940 U/L) and third week (6.643+0.802 U/L), confirming the fact that the enzyme activity in DCs was greater than in the CCs, more so in the distal (compression) than in the mesial (tension) sites. The increased ACP activity in the GCF in the distal sites of DCs reflects the biologic activity in the periodontium during orthodontic tooth movement (OTM) and could therefore serve to monitor bone turnover changes during OTM in clinical practice.
Keywords
orthodontic tooth movement; acid phosphatase; gingival crevicular fluid
Download this article as:| Copy the following to cite this article: Yezdani A. Acid Phosphatase Activity in Gingival Crevicular Fluid During Human Orthodontic Tooth Movement. Biomed Pharmacol J 2015;8(October Spl Edition) |
| Copy the following to cite this URL: Yezdani A. Acid Phosphatase Activity in Gingival Crevicular Fluid During Human Orthodontic Tooth Movement. Biomed Pharmacol J 2015;8(October Spl Edition). Available from: http://biomedpharmajournal.org/?p=3584> |
Introduction
Orthodontic tooth movement is characterized by continual bone deposition in the tension side and continual bone resorption in the compression side. However, this bone turnover is characterized by periods of activation, resorption, reversal and formation occurring after orthodontic force application. GCF constituents as diagnostic markers of active tissue destruction in periodontal diseases has been well documented 1, 2. Increases in lactic acid and citric acid during orthodontic tooth movement were reported by Miyajima et al 3 which suggested their correlation with alveolar bone resorption. Last et al 4 reported that the side to which the teeth were moved orthodontically had increased levels of chondroitin sulphate. Lowney et al 5 reported an increase in tumour necrosis factor alpha in GCF from teeth undergoing orthodontic force. Griffiths et al 6 found that in orthodontically treated teeth there was an increase in the levels of osteocalcin and piridinium cross-links of bone collagen in the GCF. Elevations in acid phosphatase levels is associated with bone resorption 7,8 and the same in alkaline phosphatase levels accompanies bone formation 9. Insoft et al 10 described the activity of acid and alkaline phosphatases in human GCF during orthodontic treatment in a longitudinal study for 3 cases. Few studies have been reported on the exclusive role of acid phosphatases in GCF to monitor the biologic process in the periodontium. The present longitudinal study monitored GCF (ACP) activity temporally and spatially during orthodontic treatment in seven human subjects with commercially available assays.
Materials and Methods
Seven orthodontic patients, 2 males and 5 females, (age range, 14 – 27 years, mean 23 years ) were included in the study. Informed consent was obtained from the patients and the protocol was viewed and approved by the Ethical Committee of Bharath University, Chennai, India.
Inclusion criteria
Good general health and oral health with full – mouth plaque score and full – mouth bleeding score less than or equal to 20%.
No use of nonsteroidal anti-inflammatory drugs a month preceding the study as also during the study period to preclude any interference with normal orthodontic tooth movement.
Fixed appliance therapy (pre adjusted edgewise appliance (MBT, 3M-Unitek; Monrovia, California), 0.022 x 0.028 – inch slot was strapped up. Transpalatal anchorage was used. Prior to canine distalization, initial aligning and leveling was completed.
Rigid oral hygiene instructions were given. In each patient, the maxillary canine on the right side was the distalized canine (DC) and was used as the experimental tooth and the contralateral canine (CC) was used as the control tooth. A continuous stainless steel ligature wire (0.010 – inch) was used to lace together passively the teeth from CC to the contralateral maxillary right lateral incisor. A nickel titanium coil spring 9 mm in length stretched from the canine hook to the hook on the maxillary right first molar tube was used to distalize the maxillary right canine on a 0.017 x 0.025 inch stainless steel archwire. Presence or absence of dental plaque (PL) and bleeding on probing (BoP) was clinically monitored before the baseline examination, during the experimental period and after 28 days.
Supragingival plaque was removed with cotton pellets and a gentle stream of air was directed towards the tooth surface for 5 seconds to dry the areas and cotton pellets were used to isolate each crevicular site included in the study. One µL of GCF was collected with a (HirschmannR microcapillary pipette, Sigma AldrichR), from both the mesial and the distal aspects of the CC and DC; before canine distalization, an hour after canine distalization and regularly every week for 4 weeks.The GCF fluid so collected was diluted to 99µL with Sorensens media containinig 0.05% bovine serum albumin in phosphate-buffered saline pH 7.0 in a plastic cuvette.The working reagent solution was prepared by dissolving 1 substrate tablet in 2.2mL buffer solution. One mL of this working reagent solution was added to 100µL of the GCF sample solution in a plastic cuvette and the ACP activity was assayed with a spectrophotometer at 300C at 405nm. The quantitative kit used was, Acid Phosphatase kit (DEA), ( pNPP Kinetic method) , Coral Clinical Systems, Volmolenheide, Belgium. Composition: Citrate Buffer 50 mmol/L pH 5.2, α-Naphthyl Phosphate > 3 mM, Fast Red TR > 1 mM and preservatives.
ACP at an acidic pH hydrolyses α-Naphthylphosphate to form α-Naphthol and Inorganic Phosphate. The α-Naphthol formed is coupled with Fast Red TR salt to form a diazo dye complex. The rate of formation of this complex is measured as an increase in absorbance at 405nm which is proportional to the ACP activity in the sample.The absorbance was converted into enzyme activity units released per minute at 300 C. Readings were noted immediately after initiation of the reaction (A1), 1 minute later (A2), 2 minutes later (A3) and 3 minutes later (A4). The summation of the changes over the 3 minutes period starting from A1 to A4 [(A2-A1) + (A3-A2) + (A4-A3)], was then calibrated and the change in absorbance was noted and designated as delta A. The mean change in absorbance per minute was calculated (delta A/min). Total acid phosphatase activity in U/L was calculated using the formula: Delta A/min x 750.
According to the readings obtained in the spectrophotometer a master chart was prepared for the enzyme activity. The mean level of acid phosphatase activity was calculated and the standard deviation of the mean values of the enzyme activity at the mesial and distal aspects of the test and control sites was determined. Independent Samples t-test and Mann-Whitney U-test was done for comparison of enzyme activity among the pre-determined intervals and the SPSS computer program version 21 was used to carry out the statistical evaluation.
Results
There was no clinically detectable movement in CCs, whereas DCs underwent a distal movement of about 1mm in the period of study of about 28 days. From the ACP activity values obtained at each site from every individual patient included in the study, the mean ACP levels in the GCF at the mesial and distal sites of the DC and the CC was calculated. (Table I).
Table I: Acid Phosphatase Activity
Summary Statistics and Comparison tests between Experimental and Control Groups
| Time | Group | ||
| Experimental | Control | ||
| Mean ± SD | Mean ± SD | ||
| Mesial | Before activation | 1.821± 0.401 | 1.821± 0.401 |
| 1 Hr. after | 1.929± 0.590 | 2.036± 0.366 | |
| 7 Days | 3.107± 0.518 | 2.464± 0.366 | |
| 14 Days | 3.643± 0.518 | 2.571± 0.401 | |
| 21 Days | 4.929± 0.401 | 2.786± 0.940 | |
| 28 Days | 4.071± 0.401 | 2.679± 0.850 | |
| Distal | Before activation | 2.357± 0.675 | 1.929± 0.590 |
| 1 Hr. after | 2.143± 1.009 | 1.607± 0.675 | |
| 7 Days | 3.964± 0.940 | 2.464± 0.567 | |
| 14 Days | 4.607± 0.802 | 3.000± 0.433 | |
| 21 Days | 6.643± 0.802 | 3.536± 0.366 | |
| 28 Days | 5.464± 0.835 | 2.679± 0.401 | |
Since the observed values of acid phosphatase activity in Gingival Crevicular Fluid (ACP in GCF) in mesial and distal sites was close to the expected values in the P-P plot, it was assumed that the said activity follows normal distribution and hence independent samples t-tests (parametric tests) were used for making comparison between control and experimental group. An attempt was also made with non-parametric test (Mann-Whitney U test) for making comparison between control and experimental group. Acid phosphatase activity in GCF was found to have similar values between experimental and control groups both in mesial and in distal sites ‘before activation’ and ‘1 hour after activation’ periods. In both the mesial and distal sites, the ACP in GCF is found to have different levels between experimental and control groups after 7 days of activation. The ACP in GCF level was found to be higher in the experimental group compared to the control group both in mesial and distal sites after 7 days of activation. A statistically significant increase in the level of ACP activity was observed on the 7th day in the distal sites of the experimental group in comparison to that of in the distal sites of the control group. The mesial sites of both the experimental and control groups were closer to the baseline values on the 7th day. A steady increase in the level of ACP activity was observed from the 7th day to the 14th day more so in the distal sites than in the mesial sites of the experimental group whereas the levels were the same in mesial and distal sites of the control group. However, there was a distinct peak in the level of ACP activity on the 21st day in the experimental group in the distal site (site of compression) and this observation was statistically significant with a drop in the level in the same site on the 28th day. (Fig.1, Fig. 2).
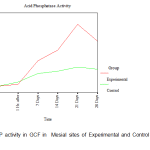 |
Figure 1: ACP activity in GCF in Mesial sites of Experimental and Control Maxillary Canines |
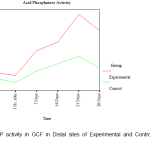 |
Figure 2: ACP activity in GCF in Distal sites of Experimental and Control Maxillary Canines |
Discussion
Animal studies have shown that the process of remodeling is more complex and it has been histologically observed that bone resorption and deposition takes place in both the compression and tension sites of the alveolar bone 11, 12. However, orthodontic tooth movement produces bone remodeling with resorption in pressure sites and deposition in tension sites 13. Early detection of periodontal disease can be effectively evaluated by the biochemical analysis of GCF 14.
The role of ACP activity in orthodontic tooth movement is interesting as it is an essential enzyme for bone resorption. Acid Phosphatase (ACP) is an enzyme of the Hydrolase class of enzymes and acts in an acidic medium. It is widely distributed and found in high concentrations in the liver, RBC’s and the prostate. Increased levels of the prostatic fraction are associated with prostatic carcinomas. Increased levels of the non prostatic fraction are associated with liver diseases, hyperparathyroidism and Paget’s disease. Hence, to avoid any ambiguity the criteria of selection of patients for the study was that they exhibit good general health.
In the present human longitudinal study, the GCF ACP activity in the DC was compared with that of CC and the results showed that the enzymatic activity was dependent on the orthodontic phase and was significantly different among DCs and CCs. Several different approaches are adopted for the collection of GCF. A method which is less disturbing to the crevicular epithelium and facilitates more rapid measurements is the placement of filter paper strips in the gingival crevice 15-17. Collection of predetermined volumes of GCF with microcapillary tubes has been reported in the literature18,19. In our study the Hirschmann microcapillary pippete was used to collect 1µLof native GCF. Care was taken not to disrupt the delicate crevicular epithelium as the microcapillary tube was passed back and forth in the gingival crevice for 10-15 minute periods. Any contamination with blood or serum was discarded and a fresh sample was taken. Acid phosphatase activity in GCF was found to have similar values between experimental and control groups both in mesial and in distal sites ‘before activation’ and ‘1 hour after activation’ periods. The ACP in GCF was found to have different levels between experimental and control groups after 7 days of activation in both the mesial and distal sites. There was an increase in the acid phosphatase level on the 7th day in the experimental group more so in the distal region (areas of compression). 20 There was a steady increase in the ACP levels and it reached peak levels on the 21st day in the distal sites of the experimental group indicating active bone resorption activity. Yokoya et al 21 too reported that osteoclasts increased upto the 7th day on the pressure side but fell rapidly by 14th day. Gingival inflammation too can cause increased ACP activity.10, 22 As the plaque and bleeding on probing score was less than 20%, it was deduced that the increase in ACP activity was due to a mechanically induced inflammation and not a bacterially induced one. The greater ACP activity values reported for the distal (compression) sites as compared to that of the mesial (tension) sites on day 7th and 21st, might be a consequence of the prevalence of bone resorption over deposition. This observation was in concurrence with other significantly reported data. 14, 23
ACP activity at the mesial and distal sites of the CC remained stable throughout the study, reiterating the fact that the distalized canine could have evoked the spike in ACP activity. This is concomittant with the reports that suggest that orthodontic forces produces distortion of the periodontal ligament extra-cellular matrix and the resultant alterations in cell shapes leads to the synthesis of inflammatory mediators, acids, extra-cellular matrix components and tissue degrading enzymes that eventually induce cellular differentiation, proliferation and remodeling.24
Since orthodontic tooth movement is a periodontal ligament phenomenon, the release of acid phosphatase in the GCF and its subsequent estimation would enable the orthodontist to monitor the biologic processes occurring during OTM, thereby serving as an invaluable diagnostic tool in clinical practice. It may be surmised that estimation of ACP activity in GCF would enable the orthodontist to deliver optimal forces and based on individual tissue responses manage the appliance effectively
Conclusions
- Significant variations in acid phosphatase level was observed in the GCF in the distal site of the distalized canine. i.e., in the areas of compression in comparison to that of the mesial site which was the area of tension.
- Increased ACP activity was observed on the distal site of the distalized canine with significant peaks on the 7th and 21st
- A relative decrease in rate of ACP activity was seen on the 14th and 28th day on the distal site of the distalized canine.
- The ACP activity on the mesial site of the distalized canine did not show as marked an increase as on the distal site as it was the area of tension.
- ACP activity on the control side showed values near to baseline scores on both the mesial and distal sites.
References
- Mc Culloch CA.Host enzymes in gingival crevicular fluid as diagnostic indicators of periodontitis .(Review).J ClinPeriodontol 1994;21:497-506.
- Lamster IB.The host response in gingival crevicular fluid: potential applications in periodontitis clinical trials (Review). J Periodontol 1992;63(12S):1117-23.
- Miyajima K, Ohno Y, Iwata T, Tanida K, Iizuka T.The lactic acid and citric acid content in the gingival fluid of orthodontic patients. Aichi Gakuin Dent Sci 1991;4:75-82.
- Last KS, Donkin C, Embery G. Glycosaminoglycans in human gingival crevicular fluid during orthodontic movement. Arch Oral Biol 1988;33(12):907-12.
- Lowney JJ, Norton LA, Shafer DM, Rossomando EF. Orthodontic forces increase tumour necrosis factor alpha in the human gingival sulcus. Am J OrthodDentofacialOrthop 1995;108:519-24.
- Griffiths GS, Moulson AM, Petrie A, James IT. Evaluation of osteocalcin and Peridinium cross-links of bone collagen as markers of bone turnover in gingival crevicular fluid during different stages of orthodontic tooth movement. J ClinPeriodontol 1988;25:492-8.
- Cohn ZA, Weiner E. The particulate hydrolasesof macrophages: I, comparative enzymology, isolation and properties.J Exper Med 1963;118:991-1008.
- BurstoneM.Histochemical demonstration of acid phosphatase activity in osteoclasts. J Histochem Cytochem 1959;7:39-41.
- Robinson R. The possible significance of hexosephophosphoric esters in ossification.Biochem J 1923;17:286-93.
- Insoft M, King GJ, Keeling SD. The measurement of acid and alkaline phosphatase in gingival crevicular fluid during orthodontic tooth movement. Am J Orthod Dentofacial Orthop 1996;109:287-96.
- King GJ, Keeling SD, Wronski TJ. Histomorphometric study of alveolar bone turnover in orthodontic tooth movement. Bone 1991;12:401-9.
- Keeling S, King G, Valdez M. Serum and alveolar bone phosphatase changes reflect remodelling during orthodontic tooth movement. Am J OrthodDentofacialOrthop 1992;103:320-6.
- Storey E.The nature of tooth movement. Am J Orthod 1973;63:292-314.
- Lilja ES, Lindskog S, Hammerstrom L. Histochemistry of enzymes associated with tissue degradation incident to orthodontic tooth movement. Am J Orthod 1983;83; 62-75.
- Lamster IB, Oshrain RL, Harper DS, Celenti RS, Hovliaras CA, Gordon JM. Enzyme activity in crevicular fluid for detection and prediction of clinical attachment loss in patients with chronic adult periodontitis: six month results. J Periodontol 1988;59(8)516-23.
- Lamster I, Oshrain R, Florella L, Celeuti R, Gordon J. A comparison of four methods of data presentation for lysosomal enzyme activity in gingival crevicular fluid. J ClinPeriodontol 1988;15:317-52.
- Binder T, Goodson J, Socransky S. Gingival fluid levels of acid and alkaline phosphatase. J Periodontal Res 1987;22:14-9.
- Krasse B, Egelberg J.The relative proportions of sodium, potassium and calcium in gingival pocket fluid.ActaOdontolScand 1962;20:143-52.
- Kaslick RS, Chasens AJ, Weinstein D, Waldman R, Pluhar T, Lazarra R.Quantitative analysis of sodium, potassium and calcium in gingival fluid from gingiva with varying degrees of inflammation. J Periodontol 1970;41:93-7.
- Takimoto K, Deguchi T, Mori M. Histochemical detection of acid and alkaline phosphatises in periodontal tissues after experimental tooth movement. J Dent Res 1968;47:340.
- Yokoya K, Sasaki T, Shibasaki Y. Distributional changes of osteoclasts and pre-osteoclastic cells in periodontal tissues during experimental tooth movement as revealed by quantitative immunohistichemistry of H (+)-ATPase . J Dent Res 1997;76:580-7.
- Ishikawa I, Cimasoni G, Held AJ. Alkaline phosphatase in human gingival fluid and it’s relation to periodontitis. Arch Oral Biol 1970;15:1401-4.
- Engstrom C, Granstrom G, Thilander B. Effect of orthodontic force on periodontal tissue metabolism. Am J Orthod Dentofac Orthop 1988;93:486-95.
- Tsatala SK, Kaklamanos EG, TsalikisLazaros.Effects of orthodontic treatment on gingival crevicular fluid flow rate and composition: Clinical implications and applications.Int J Adult Orthod Orthognathic Surg 2002;17:191-205.







