Hamid Haghighatfard1,2, Nader Mansour Samaei1, Toraj Farazmandfar1, Mazdak Ganjalikhani Hakemi3, Ahad Yamchi4 , Farhad Jadidi-Niaragh5,6 and Yaghoub Yazdani*2,1
1Department of Medical Biotechnology, Faculty of Advanced Medical Technologies, Golestan University of Medical Sciences, Gorgan, Iran. 2Infectious Diseases Research Center , Golestan University of Medical Sciences, Gorgan, Iran. 3Cellular and Molecular Immunology Research Center, Isfahan University of Medical Science, Isfahan, Iran. 4Department of Plant Biotechnology, Gorgan University of Agricultural Science and Natural Resources, Gorgan, Iran. 5Department of Immunology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran. 6Department of Immunology, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran.
DOI : https://dx.doi.org/10.13005/bpj/876
Abstract
Angiogenesis is very important in cancer growth and metastasis. Basic fibroblast growth factor (bFGF) as one of the most important angiogenesis factors is an attractive target for cancer vaccine. Due to low immunogenicity, it cannot stimulate an effective immune response. Theoretically, pseudomonas exotoxin (PE) as a potent immunogenic carrier protein when fused to low immunogenic antigens such as bFGF significantly increased immunogenicity of it. In this study, we tried to molecular cloning and expression of bFGF conjugated with immunodominant domains of pseudomonas exotoxin. The coding sequence of fusion protein composed of bFGF linked to PE domains 1b and 2 using EAAAK poly linker. The KDEL sequence was also used in C-terminal coding sequence. It was synthesized and expressed using recombinant DNA technology in the bacterial expression system. Expression of recombinant protein verified using SDS-PAGE and western blot analyses. Finally, it purified using Ni-affinity chromatography. The band close to 37 kDa in SDS-PAGE and western blot analyses was aligned completely to designed sequence. Purified recombinant protein also showed as a clear single band near to 37 kDa.
Keywords
Basic fibroblast growth factor (bFGF); Cloning; Pseudomonas exotoxin (PE)
Download this article as:| Copy the following to cite this article: Haghighatfard H, Samaei N. M, Farazmandfar T, Hakemi M. G, Yamchi A, Jadidi-Niaragh F, Yazdani Y. Molecular Cloning and Expression of Novel Fibroblast Growth Factor-2 Conjugated with Immunodominant Domains of Pseudomonas exotoxin. Biomed Pharmacol J 2015;8(2) |
| Copy the following to cite this URL: Haghighatfard H, Samaei N. M, Farazmandfar T, Hakemi M. G, Yamchi A, Jadidi-Niaragh F, Yazdani Y. Molecular Cloning and Expression of Novel Fibroblast Growth Factor-2 Conjugated with Immunodominant Domains of Pseudomonas exotoxin. Biomed Pharmacol J 2015;8(2). Available from: http://biomedpharmajournal.org/?p=6177 |
Introduction
Angiogenesis is formation of new blood vessels and is fundamental mechanism in numerous physiological and pathological processes such as embryonic development, wound healing and tumor metastasis 1,2. Basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) are potent angiogenic factors that induce tumor growth 3. Theoretically, inhibition of angiogenic factors can inhibit tumor metastasis. Targeted therapies involve production of components such as monoclonal antibodies4. Today, a humanized anti-VEGF monoclonal antibody ( Avastin) has been approved by the FDA for treatment of colorectal cancer5. More studies showed that prolonged use of this drug may be lead to drug resistance6. Detailed investigations indicated that this resistance created by bFGF effects on endothelial cells 7. Moreover, bFGF is one of the most important regulators of human embryonic stem cell (hESC) 8. Thus, it can be used as a good target for cancer therapy. The bFGF has 155 amino acids length and has a molecular weight (MW) of 17.2 kDa 9. Due to low immunogenicity and short half-life, it cannot stimulate an effective immune response 10. Several approaches have been developed to potentiate immune response to low immunogenic antigens such as adjuvant and potent antigens as a carrier 11. The PE domains 1b and 2 are one of the potent antigen carrier which could significantly increase immunogenicity of low immunogenic antigens 12. Moreover, PE when fused to a weak tumor antigens stimulate a protective cytotoxic T lymphocytes (CTL) response to heterologous antigen 13. The endoplasmic reticulum (ER) is where the major histocompatibility complex (MHC) class I molecules are loaded with antigens. Most of recombinant proteins are degraded in the cytoplasm and few epitopes reach to the ER. Theoretically, targeting of this anigens with an ER retention signal (KDEL sequence) could induce potent immune response 14.
In this study, cloning and expression and purification of bFGF conjugated with immunodominant domain of PE was done. For increasing CTL response, the KDEL signal peptide in triplicate was also used in designed coding sequence. Fusion protein produced using recombinant DNA technology in the bacterial expression system. Software analysis and protein expression analysis showed large scale production of recombinant fusion protein.
Materials and Methods
Designing the coding sequence and construction of expression vector
The coding sequence of fusion protein designed as a poly his-tag sequence at N-terminal of fusion protein- the enterokinase cut site –coding sequence of bFGF – rigid poly linker, (EAAAK) 4 – coding sequence of domain 1b and 2 of PE and (KDEL) 3 sequence. Finally, the NcoI and XhoI restriction site were introduced at the two ends of designed coding sequence. According to bacterial expression system, using bioinformatics software such as DNA2 and I-TASSER, the coding sequence was optimized. Prediction of secondary structure was assessed with online PSIPRED program (http://bioinf.cs.ucl.ac.uk/psipred). Designed coding sequence was purchased in the pUC57cloning vector (shine gene).
Then, expression vectors (pET28a) and cloning vector (pUC57) were digested by NcoI and XhoI restriction enzymes. Linearized expression vector and coding sequence extracted from agarose gel utilizing miniprep kit (Qiagen). Ligation of coding sequence into linearized expression vectors was done by T4 DNA ligase. The colony PCR test using forward and reverse T7-promoters and DNA sequencing were used for confirmation the correct insertion into the cloning site of expression vectors.
Recombinant protein expression and purification
The E. coli strains BL21 (DE3) was used for protein expression. Designed expression vector introduced into the BL21 bacterial using heat shock at 42oC. The optimization of protein expression was performed by monitoring the total protein expression. Briefly, overnight cultures of transformed bacteria were inoculated into 250 ml of LB broth containing 30 μg/ml of kanamycin. It incubated at 37oC with shaking at 130 rpm until the OD600 reached to 0.8. Expression of recombinant proteins was induced with 1 mM IPTG and was monitored at various incubation times (4, 8 and 16 hours). Total protein were assessment by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE, 12%). For Western blot analysis, total proteins were transferred to a nitrocellulose membrane at 400 mA for 45 minute using an electroblot system. Membranes were then washed extensively with PBS buffer (0.15M, pH 7.4) and blocked with 3% of skim milk. The membranes were shacked for 1.5 hour at room temperature with anti- bFGF monoclonal antibody (Abnova) at final dilution of 1:500 in PBS buffer containing 3% of skim milk. After stringent washing using PBS-tween- skim milk solution (0.05% tween, 3% of skim milk), the membranes were incubated with 1:1000 HRP conjugated sheep anti mouse antibody (Sigma) for 1.5 hour. After washing the membranes, recombinant protein was detected using DAB as the substrate for HRP and the appearance of brown colored band on the membrane confirmed recombinant protein expression 15. For purification of recombinant fusion protein, the Ni NTA agarose was used according to manufacture instruction (Qiagen). The condition of our purification method was reducing.
Results and Discussion
Cancer immunotherapy is one the most intrinsic aspect in cancer therapy 16. Utilization of humoral and cellular immune elements is useful in this strategy 17,18. In immunotherapy, more detailed recognition of target (cancer cell) is necessary. The process by which normal cells become malignant is not well known, but the sequential of mutations were seen in the genome of cancer cells. Although tumor mass generate from a unique mutant cancer cell, but cancer cells have different genotype and phenotype 19. The best approach in recognition and manipulation of cancer cells is selecting immunogenic markers that have not mutations, over expression in all cancer cells and not expressed in normal cells, or have low expression 20.
New growth in the vascular network is important for proliferation and metastasis of cancer cells. Therefore, targeting of this process may be useful in treatments of cancer patients 21. Angiogenesis is regulated by activator molecules such as VEGF and bFGF 3 , so anti-angiogeic treatments may be useful in cancer therapy.
The bFGF has low immunogenicity and short half-life in blood circulation, thus it cannot stimulate an effective immune response 10.Thus, increasing of its immunogenicity may be useful in controlling of cancer cells. Domain 1b and 2 of the PE is one of the potent antigenic carrier which could significantly increase immunogenicity of low immunogenic antigens 12.
In addition to increasing immunogenicity of low immunogenic antigens , PE toxin was also used for direct killing target cells such as cancer cells. Gawlak et al expressed recombinant fusion protein composed of bFGF and PE (toxin parts) that contain domain II and III in bacterial expression system. Recombinant fusion protein has considerable activity on inhibition of different cancer cell line22.
The first step in evaluation of new designed protein is large scale production of it. There are different approaches for production of designed proteins such as chemical synthesis and using biological expression system 15,23. Since post-translational modification of designed fusion protein is not necessary for developing of linear epitops and considerable part of designed fusion protein has bacterial source (PE), bacterial expression system was used for production of designed protein. Moreover, bacterial expression provides an economical method for production recombinant proteins and requires minimal technical specialty 24. In bacterial expression system, the pET Expression System is the best option for production of recombinant proteins. The pET28 vector enables the quick production of a large scale production of recombinant protein 24. In this expression system, the his tag sequences facilitate purification of target protein and kanamycin resistance gene lead to easy screening 25.
In this study, we tried to produce a new fusion protein that consists of bFGF and immunodominant part of PE (domain1b and 2) as carrier protein. In designated coding sequence, different part of recombinant fusion protein had logic and specific location. His tag sequence introduce at N-terminal for affinity chromatography 26 and the KDEL sequence at C-terminal for high loading of desired epitop in MHC class I grove and lead to activation of CTL response against bFGF 27. For avoidance of overlapping determinants, the rigid poly linker with alpha helix structure was used between to mentioned proteins 28. Bioinformatics prediction of codon adaptation index (CAI) and GC content showed that these parameters after optimization reached to 0.83 (CAI) and 54.7% for GC content. Prediction of secondary structure with PSIPRED program verifies rigid linker can be formed an alpha helix structure. After optimization and synthesis, coding sequence subcloned into expression vectors pET28. Investigation of colony PCR by T7-promoter showed a clear band near to 1200 bp that has similar size with designed coding sequence (Figure 1). Presentation and orientation of different part of coding sequence were also approved by DNA sequencing.
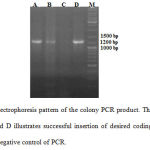 |
Figure 1: Agarose gel electrophoresis pattern of the colony PCR product. |
Prediction of three-dimensional structure of fusion protein with I-TASSER software showed that PE and bFGF are well separated from each other with rigid poly linker and do not interact with each other.
Expression of recombinant fusion protein was induced with IPTG. Expression of recombinant fusion protein was investigated by SDS-PAGE and western blot analyses. Assessment of it in transformed BL21 bacteria revealed a band close to 38 kDa in SDS-PAGE that was aligned completely to the expected recombinant protein (Figure 2). Lane A in figure 2 also indicated purification of recombinant protein was done perfectly. Figure 3 showed the result of western blot analysis. The band approximately close to 38 kDa confirmed that expressed protein is designed recombinant fusion protein.
 |
Figure 2: Profile of protein expression in transformed BL21 bacteria. |
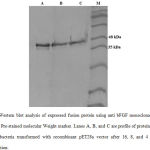 |
Figure 3: Western blot analysis of expressed fusion protein using anti bFGF monoclonal antibody. |
According to our knowledge, this study is the first work that considered molecular cloning and expression of bFGF conjugated with immunodominant domain of PE (domain1b and 2) using rigid linker in bacterial expression system.
Acknowledgements
This study is MS thesis and funded by Golestan University of Medical Sciences. Award number is 35/2068.
References
- Carmeliet,P. Angiogenesis in health and disease. Nat.Med., 2003;9(6):653-60.
- Denekamp,J. Vascular endothelium as the vulnerable element in tumours. Acta Radiol.Oncol., 1984;23(4):217-25.
- Nillesen,S.T.,Geutjes,P.J.,Wismans,R.,Schalkwijk,J.,Daamen,W.F., van Kuppevelt,T.H. Increased angiogenesis and blood vessel maturation in acellular collagen-heparin scaffolds containing both FGF2 and VEGF. Biomaterials, 2007;28(6):1123-31.
- Yazdani,Y.,Roohi,A.,Khoshnoodi,J., Shokri,F. Development of a sensitive enzyme-linked immunosorbent assay for detection of hepatitis B surface antigen using novel monoclonal antibodies. Avicenna.J.Med.Biotechnol., 2010;2(4):207-14.
- Ferrara,N.,Hillan,K.J., Novotny,W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem.Biophys.Res.Commun., 2005;333(2):328-35.
- Casanovas,O.,Hicklin,D.J.,Bergers,G., Hanahan,D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell, 2005;8(4):299-309.
- Rusnati,M..,Presta,M. Fibroblast growth factors/fibroblast growth factor receptors as targets for the development of anti-angiogenesis strategies. Curr.Pharm.Des, 2007;13(20):2025-44.
- 8. Dvorak,P.,Dvorakova,D., Hampl,A. Fibroblast growth factor signaling in embryonic and cancer stem cells. FEBS Lett., 2006;580(12):2869-74.
- Prats,H.,Kaghad,M.,Prats,A.C.,Klagsbrun,M.,Lelias,J.M.,Liauzun,P.,Chalon,P.,Tauber,J.P., Amalric,F.,Smith,J.A., . High molecular mass forms of basic fibroblast growth factor are initiated by alternative CUG codons. Proc.Natl.Acad.Sci.U.S.A, 1989;86(6):1836-40.
- Zhang,H.L.,Yuan,C.,Zhang,D.M.,Shi,H.S.,Li,M.,Luo,Z.C.,Wan,Y.,Lu,L.,Luo,S.T., Yang,L. A novel combined conjugate vaccine: enhanced immunogenicity of bFGF with CRM197 as a carrier protein. Mol.Med.Rep., 2011;4(5):857-63.
- Garcia,A..,De Sanctis,J.B. An overview of adjuvant formulations and delivery systems. APMIS, 2014;122(4):257-67.
- Lippolis,J.D.,Denis-Mize,K.S.,Brinckerhoff,L.H.,Slingluff,C.L.,Jr.,Galloway,D.R., Engelhard ,V.H. Pseudomonas exotoxin-mediated delivery of exogenous antigens to MHC class I and class II processing pathways. Cell Immunol., 2000;203(2):75-83.
- Becerra,J.C.,Arthur,J.F.,Landucci,G.R.,Forthal,D.N., Theuer,C.P. CD8+ T-cell mediated tumor protection by Pseudomonas exotoxin fused to ovalbumin in C57BL/6 mice. Surgery, 2003;133(4):404-10.
- Loera-Arias,M.J.,Martinez-Perez,A.G.,Barrera-Hernandez,A.,Ibarra-Obregon,E.R.,Gonzalez-Saldivar,G.,Martinez-Ortega,J.I.,Rosas-Taraco,A.,Villanueva-Olivo,A.,Esparza-Gonzalez, S.C.,Villatoro-Hernandez,J.,Saucedo-Cardenas,O., Montes-de-Oca-Luna,R. Targeting and retention of HPV16 E7 to the endoplasmic reticulum enhances immune tumour protection. J.Cell Mol.Med., 2010;14(4):890-4.
- Yazdani,Y.,Sadeghi,H.,Alimohammadian,M.,Andalib,A.,Moazen,F., Rezaei,A. Expression of an innate immune element (mouse hepcidin-1) in baculovirus expression system and the comparison of its function with synthetic human hepcidin-25. Iran J.Pharm.Res., 2011;10(3):559-68.
- Rosenberg,S.A.,Yang,J.C., Restifo,N.P. Cancer immunotherapy: moving beyond current vaccines. Nat.Med., 2004;10(9):909-15.
- Beavis,P.A.,Slaney,C.Y.,Kershaw,M.H.,Neeson,P.J., Darcy,P.K. Enhancing the efficacy of adoptive cellular therapy by targeting tumor-induced immunosuppression. Immunotherapy., 2015;7(5):499-512.
- Reuschenbach,M.,von Knebel,D.M., Wentzensen,N. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol.Immunother., 2009;58(10):1535-44.
- Bertram,J.S. The molecular biology of cancer. Mol.Aspects Med., 2000;21(6):167-223.
- Jager,E.,Jager,D., Knuth,A. Antigen-specific immunotherapy and cancer vaccines. Int.J.Cancer, 2003;106(6):817-20.
- Nishida,N.,Yano,H.,Nishida,T.,Kamura,T., Kojiro,M. Angiogenesis in cancer. Vasc.Health Risk Manag., 2006;2(3):213-9.
- Gawlak,S.L.,Pastan,I., Siegall,C.B. Basic fibroblast growth factor-Pseudomonas exotoxin chimeric proteins; comparison with acidic fibroblast growth factor-Pseudomonas exotoxin. Bioconjug.Chem., 1993;4(6):483-9.
- Yazdani,Y.,Keyhanvar,N.,Kalhor,H.R., Rezaei,A. Functional analyses of recombinant mouse hepcidin-1 in cell culture and animal model. Biotechnol.Lett., 2013;35(8):1191-7.
- Khow,O..,Suntrarachun,S. Strategies for production of active eukaryotic proteins in bacterial expression system. Asian Pac.J.Trop.Biomed., 2012; 2(2):159-62.
- Costa,S.,Almeida,A.,Castro,A., Domingues,L. Fusion tags for protein solubility, purification and immunogenicity in Escherichia coli: the novel Fh8 system. Front Microbiol., 2014;5:63.
- Yazdani,Y.,Keyhanvar,N., Tabaraei,A. Cloning and functional assessment of the recombinant human hepcidin-25 in the baculovirus expression system. Braz.Arch.Biol.Technol., 2015;58(1):90-5.
- Cabrera,M.,Muniz,M.,Hidalgo,J.,Vega,L.,Martin,M.E., Velasco,A. The retrieval function of the KDEL receptor requires PKA phosphorylation of its C-terminus. Mol.Biol.Cell, 2003;14(10):4114-25.
- Chen,X.,Zaro,J.L., Shen,W.C. Fusion protein linkers: property, design and functionality. Adv.Drug Deliv.Rev., 2013;65(10):1357-69.








