Manuscript accepted on :
Published online on: 26-12-2015
Plagiarism Check: Yes
Tahoora Shomali1*, Alireza Rafati1, Mehdi Fazeli1, Mohammad Kamalpour1, Akbar Safaei2
1Division of Pharmacology and Toxicology, Department of Basic Sciences, School of Veterinary Medicine, Shiraz University, Shiraz, Iran 2Department of Basic Sciences, Division of Pathology, Shiraz Medical School, Shiraz University of Medical Sciences, Shiraz, Iran
DOI : https://dx.doi.org/10.13005/bpj/811
Abstract
The hydroxy-carboxylic acid 2 (HCA2) receptors have attracted new interests as the target of antidyslipidemic drug niacin. The presence of mRNA for these receptors has been previously described in rats' femur. The present study aimed at detection of cell types that express HCA2 receptor, their relative receptor density and effect of niacin administration in proximal femur of rats. Methods: Growing male rats kept untreated or received niacin (200 mg/kg/day orally). After 4 weeks, proximal femur was evaluated. Results: Despite a minor decrease in histomorphometric parameters in niacin group, no deleterious effect was observed. In immunohistochemical evaluation, many different cells showed HCA2 receptor expression with the highest receptor density in marrow cells followed by endothelial cells, adipocytes, fibroblasts, striated muscle, osteoblasts, osteocytes, chondroblasts and finally chondrocytes. Osteoclasts were moderately positive. Niacin reduced receptor density in adipocytes, fibroblasts, striated muscle, osteoblasts and osteocytes. Receptor density of marrow cells, chondroblasts and endothelial cells remained statistically the same and in chondrocytes appreciably increased. Immunohistochemical reactivity of the total slide (marrow and trabeculae) as well as mRNA level of HCA2 receptor in qPCR evaluation remained statistically the same. Conclusions: In conclusion, the study showed the presence of HCA2 receptors in bone and cartilage cells with higher expression in osteoblasts and chondroblasts than their mature forms. Moreover, niacin administration resulted in cell type-dependent changes in receptor density of the cells present in the proximal femur of rats.
Keywords
HCA2 receptor; bone; immunohistochemistry; qPCR; localization; density; rat
Download this article as:| Copy the following to cite this article: Shomali T, Rafati A, Fazeli M, Kamalpour M, Safaei A. Localization, Cellular Density and Gene Expression of HCA2 Receptors in Proximal Femur of Normal and Niacin-Treated Rats. Biomed Pharmacol J 2015;8(2) |
| Copy the following to cite this URL: Shomali T, Rafati A, Fazeli M, Kamalpour M, Safaei A. Localization, Cellular Density and Gene Expression of HCA2 Receptors in Proximal Femur of Normal and Niacin-Treated Rats. Biomed Pharmacol J 2015;8(2).Available from: http://biomedpharmajournal.org/?p=3228> |
Introduction
The hydroxy-carboxylic acid (HCA) receptors including HCA1, HCA2 and HCA3 (previously known as GPR81, GPR109A and GPR109B, respectively, or as the nicotinic acid receptor family), are G protein-coupled receptors with hydroxy-carboxylic acids, as their endogenous ligands. These receptors are predominantly expressed on adipocytes and mediate the inhibition of lipolysis by coupling to Gi–type proteins (1). Ketone body 3-hydroxy-butyrate is endogenous ligand for HCA2 receptor and this receptor is the most extensively studied among the three subtypes, since it is the target of the antidyslipidemic drug nicotinic acid (or niacin) (2-4).
HCA2 is highly expressed in human and murine white and brown adipose tissue, moreover; various immune cells (5, 6), keratinocytes (7), and retinal pigment epithelium as well as intestinal epithelium (8, 9) express this receptor. In 2011, Titgemeyer et al. identified HCA2 receptor in bovine liver, muscle and brain (10). The tissue distribution of hamster and guinea pig HCA2 receptor mRNA has also been determined by quantitative real-time PCR by Torhan et al., 2007 (11). These researchers reported that the expression is fairly widespread, with highest expression in lung, spleen, testis and adipose tissues of hamsters and spleen, lung, stomach, skeletal muscle, ovary and adipose tissues of guinea pigs.
Regarding the wide spread use of rats as an animal model in experimental studies and the lack of knowledge about the tissue distribution pattern of HCA2 receptors in this laboratory animal, we previously screened different organs of rats for HCA2 receptor mRNA by PCR method which showed the presence of its mRNA in different organs including proximal epiphyseal and metaphyseal areas of femur (12). Although there is considerable knowledge about the effect of niacin on bone marrow cells (13-16), information about the localization of its receptors especially in trabecular bone cells is scares. It has been shown that niacin can lower trabecular number and increase trabecular separation in femoral metaphysis of methylprednisolone-treated rats (17), which potentiates the possibility for the presence of niacin receptors in bone cells. In the present study we report immunohistochemical detection of the cell types that express HCA2 receptor and relative receptor density in different cells as well as the effect of niacin administration on gene and protein expression of the receptor in proximal femoral bone of rats.
Materials and Methods
Animals and experimental design
Sixteen growing male Sprauge Dawley rats with a mean body weight of 150 g were used. Rats were acclimatized for 10 days to the ambient conditions (temperature about 23°C and a 12h/12h, light/dark cycle) and had free access to commercial chew pellets and tap water. After adaptation period, animals were randomly divided into 2 equal groups and treated as follows: 1- normal control group (CG) that left without treatment (normal saline at the same volume to that of niacin suspension administered to a rat with the same weight) and 2- niacin group (NG) which received 200 mg/kg/day niacin (Novin Kavosh Mamtir Co., Iran) (suspended in normal saline) by oral gavage. After 4 weeks, all rats were sacrificed under deep ether anesthesia and immediately right and left femoral bones were removed for qPCR and histological examination, respectively, qPCR samples were kept in -70ºC until use.
All procedures used in the present study are in accordance with our institutional ethical guidelines for care and use of animals in experiments which are compatible with European convention for the protection of vertebrate animals used for experimental and other scientific purposes.
Histomorphometric study
H and E-stained slides from proximal epiphysis and metaphysis of left femoral bones were blindly evaluated qualitatively and quantitatively for histomorphometric parameters including epiphyseal trabecular width (epi. Tb.Wi), metaphyseal trabecular width (Mt. Tb.Wi),
epiphyseal bone area/tissue area (epi. B.Ar/T.Ar) and metaphyseal bone area/tissue area (Mt. B.Ar/T.Ar) by using Ziess Axiovision LE 4.8.2 software. All parameters were measured in a central zone of epiphysis or secondary spongiosa in metaphysis. The mean value of ten measurements of each parameter in each sample was calculated. The nomenclature of parameters is in compliance with ASBMR histomorphometry nomenclature committee (18).
Immunohistochemical evaluation
Four μm-paraffin sections from proximal epiphysis and metaphysis of left femoral bones were obtained in median plate after formic acid-sodium citrate decalcification. Endogenous peroxidases were inactivated by 3 minute incubation in 3% hydrogen peroxide in methanol. The primary antibody for Immunohistochemical staining was rabbit polyclonal GPR109A antibody (1:300, incubation for 60 min at room temperature) which reacts with rat HCA2 receptor, prepared by Biorbyt Ltd., United Kingdom. Visualization was made by EnVision™ Detection Systems Peroxidase/DAB for Rabbit/Mouse antibodies (1:2, 30 and 5 min incubation at room temperature for secondary antibody and DAB chromogen, respectively) (Dako, Denmark). Then, slides were counter stained with H and E. Negative controls were treated with secondary antibody only. Fat tissue was used as a positive control for presence of the receptor.
Color images of the slides under light microscope were prepared and blindly evaluated by Softonic® Image Analyzer software with regard to hue, saturation and brightness (HSB), which is inversely correlated to receptor density (19). For quantitative determination of cellular density of the receptor, at least 5 cells of each type in each slide were randomly assayed and the mean HSB value was calculated. HSB value of the total slide (including bone marrow and trabeculae) was also determined.
RNA isolation and real time qPCR
Samples weighing 50-100 mg, from proximal epiphysis and metaphysis of right femoral bones (including bone marrow and trabeculae) were powdered manually in liquid nitrogen and total RNA was extracted by using AccuZol™ Total RNA Extraction Solution (Bioneer, Korea), as described by manufacturer and treated with DNase I (Fermentas Inc., United States). First strand cDNA was synthesized from 8μl of total RNA by using RocketScriptTM Cycle RT PreMix (Bioneer, Korea). The real-time PCR contained 3μl of cDNA, 0.5μl forward and reverse primers, 6.25μl of AccuPower® GreenStarTM qPCR PreMix (Bioneer, Korea) and 2.25μl distilled water using MJ Mini™Thermal Cycler and MiniOpticon™Real-Time PCR system (Bio-Rad, United States). The following primers were used: GPR109A/HCA2, forward: 5´-CGGTGGTCTACTATTTCTCC-3´, reverse: 5´-CCCCTGGAATACTTCTGATT-3´; GAPDH, forward: 5´-AAGGATACTGAGAGCAAGAG-3´; GAPDH, reverse: 5´-TGATGGTATTCGAGAGAAGG-3´, which yielded 159 and 154 bp products, respectively. The annealing temperature was 55ºC for 30 sec. Melting curves were produced for HCA2 receptor and GAPDH PCR products. Quantification was performed by the comparative Ct method with GAPDH used as internal control.
Statistical Analysis
Data were presented as mean±SD and analyzed by independent samples t-test. A significance level of p<0.05 was considered for all comparisons.
Table 1: Trabecular bone histomorphometric parameters (mean±SD) of control and niacin-treated rats (n=8 each)
| Control group | Niacin-treated group | |
| epi. Tb.Wi (µm) | 86.2±14.9 | 73.5±14.6 |
| Mt. Tb.Wi (µm) | 40.8±6.2 | 36.7±4.9 |
| epi. B.Ar/T.Ar (%) | 32±6 | 30±5.1 |
| Mt. B.Ar/T.Ar (%) | 35.6±5.7 | 32.2±4.8 |
No significant difference was observed between groups (p>0.05).
epi. Tb.Wi: epiphyseal trabecular width; Mt. Tb.Wi: metaphyseal trabecular width; epi. B.Ar/T.Ar: epiphyseal bone area/tissue area and Mt. B.Ar/T.Ar: metaphyseal bone area/tissue area.
Table 2: Cellular density of HCA2 receptor in different cells based on HSB value (mean±SD) in control and niacin-treated groups
| Cell type
Groups |
MC | CB | CC | OB | OC | FB | AC | VEC | SMC | TS |
| Control | 83.6±
11.5 |
149±
13.1 |
166±
5.34 |
141±
1.29 |
143±
1.29 |
136±
10.8 |
130±
13.0 |
128±
5.65 |
139±
2.14 |
147±
9.02 |
| Niacin-treated | 81.5±
10.2 |
139±
12.3 |
156±
4.09* |
175±
0.706* |
172±
2.14* |
181±
7.18* |
164±
25.1* |
134±
3.85 |
172±
0.898* |
162±
16.2 |
MC: marrow cells, CB: chondroblasts, CC: chondrocytes, OB: osteoblasts, OC: osteocytes, FB: fibroblasts, AC: adipocytes, VEC: vascular endothelial cells, SMC: striated muscle cells and TS: total slide.
Asterisk sign shows significant difference with control group (p<0.05).
HSB value is inversely correlated with receptor density.
Results
Histological evaluation
Qualitative evaluation of slides revealed no sign of detrimental effect of niacin on bone structures or haematopoietic cells. Although all measured histomorphometric parameters were slightly lower in niacin-treated group, no significant difference was found (p>0.05). Data are presented in table 1.
Localization and cellular density of HCA2 receptor
Many different cell types showed HCA2 receptor expression including many bone marrow cells, chondroblasts, chondrocytes, osteoblasts and osteocytes, fibroblasts, adipocytes, vascular endothelial cells (vascular smooth muscle cells showed no reactivity) and even a few striated muscle cells which were stuck to bones in histological slides. Osteoclasts were rarely present in sections and were moderately positive (Fig. 1).
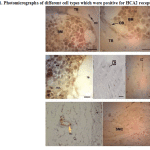 |
Figure 1: Photomicrographs of different cell types which were positive for HCA2 receptor |
Quantitative examination revealed that in normal rats; positive bone marrow cells had the highest receptor density among different cell types followed by endothelial cells, adipocytes, fibroblasts, striated muscle cells, osteoblasts, osteocytes, chondroblasts and finally chondrocytes. The comparison between two groups with regard to receptor density in different cell types showed that niacin administration has significantly reduced receptor density in fat cells (p=0.028), fibroblasts, striated muscle cells, osteoblasts and osteocytes (p=0.000 for all comparisons), while no significant difference was observed in HCA2 receptor density of marrow cells, chondroblasts and endothelial cells between two groups (p>0.05). Receptor density of chondrocytes was appreciably increased due to niacin administration (p=0.007).
On the other hand, evaluation of HSB value in the whole section which included both bone marrow and trabeculae, revealed no significant difference between control and NG (p>0.05). Data are summarized in table 2.
HCA2 receptor mRNA expression
Melting curves for GAPDH and HCA2 receptor qPCR products are shown in fig 2. Single product-specific melting temperatures were 85.2 ºC and 85.6 ºC for HCA2 receptor and GAPDH, respectively.
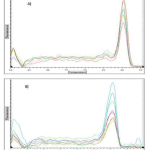 |
Figure 2: Melting curves for HCA2 receptor (A) and GAPDH (B) qPCR products |
mRNA level of HCA2 receptor genes as determined by ∆∆Ct method was 3.50±1.61 in control group and 4.21±1.93 in NG. No significant difference was observed between two groups.
Discussion
Bones are dynamic structures with different homeostatic functions including protection of vital organs and a pivotal role in locomotion as well as embracing haematopoietic cells and even working as an endocrine organ (20). Different cell types are present in bone with close or contrary functions. It is very interesting that bone and lipid metabolism is closely related in a way that lipids have a role in controlling bone cellular balances including adipocytogenesis/osteoblastogenesis and osteoblastogenesis/osteoclastogenesis. Fatty acids, cholesterol, phospholipids and several endogenous metabolites influence bone cell survival and functions, the bone mineralization process, and critical signaling pathways. Thus, they can be regarded as regulatory molecules important in bone health (21).
G protein-coupled receptors comprise a large family of cell-surface receptors with different intracellular signaling cascades and high diversity. They are very important both physiologically and pharmacologically since they are the target of a major group of clinically used drugs. Detection of these receptors, clarifying their roles and characterization of the cell types that express a specific subtype is important for finding new treatment strategies. HCA2 receptors are G protein-coupled receptors which have attracted new interests due to their role in lipid metabolism as the target of antidyslipidemic drug niacin accompanied by other functions such as anti-inflammatory effects in different organs (22).
As previously stated tissue distribution of these receptors has been investigated in different species including cattle (10), hamsters and guinea pigs (11), rats (12) and recently cats (23). All of these researchers have shown an extensive presence of HCA2 receptors in different tissues. We previously detected mRNA for this receptor in proximal epiphyseal and metaphyseal areas of femur in rats (12); however we could not characterize the specific cell type that expressed receptor, receptor density in different cells and possible changes in receptor population due to repeated dose administration of niacin which together motivated to perform the present study.
Our study clearly showed that bone and cartilage cells express the receptor and mature cells (osteocytes and chondrocytes) have less receptor population than active “blastic” forms. Since we worked on growing rats with “modeling” as the dominant process in the bone, we could rarely observe osteoclasts. These cells were moderately positive for the receptor, however due to their low population, we were not able to quantitatively evaluate their receptor density.
Niacin administration led to receptor down-regulation in osteoblasts and osteocytes while chondroblasts were not appreciably affected and interestingly chondrocytes showed higher population of receptors in niacin-treated rats. Although receptor up-regulation due to agonist administration is not as common as down-regulation; however there are previous reports of this paradox; for instance in 1990, Wonnacott described the upregulation of nicotinic acetylcholine receptor by nicotine (24). Up-regulation of β3-adrenergic receptors by long-term agonist exposure (25) and up-regulation of some of the somatostatin receptor subtypes after prolonged agonist treatment (26) are other examples.
Although we found changes in receptor population in different cells due to niacin administration, no significant change in HCA2 receptor mRNA content was observed by qPCR. This observation may be described by the fact that both bone marrow and trabeculae were present in samples used for qPCR and as we previously stated, different cell types had responded differently to niacin administration with regard to receptor density (down-regulation, up-regulation and even no change at all) and the output of all these was measured in qPCR, for example niacin administration had no significant effect on receptor population in marrow cells that make a substantial part of the samples. Consistent with this, reactivity of total slides (bone marrow and trabeculae) in immunohistochamical evaluation was statistically the same between the two groups. Therefore it seems that evaluation of HCA2 receptor mRNA expression needs to be more precisely assayed with regard to specific cell types.
In histological study, although we observed a minor decrease in histomorphometric parameters, there was no deleterious effect on bone structure due to niacin administration. It should be mentioned that the course of our study was relatively short and with regard to the fact that administration of niacin, resulted in down-regulation of HCA2 receptors in osteoblasts and osteocytes; we cannot rule out that the physiological function of these cells and their response to 3-hydroxy-butyrate as the endogenous ligand for HCA2 receptors is not affected which obviously needs more sophisticated experiments.
Conclusions
In conclusion, the study showed the presence of HCA2 receptors in bone and cartilage cells with higher expression in osteoblasts and chondroblasts than their mature forms. Moreover, niacin administration can result in cell type-dependent changes in receptor density of the cells present in the proximal femur of rats.
Acknowledgements
Funding for the study was provided by Shiraz University, School of Veterinary Medicine.
References
- Blad, C.C., Ahmed, K., Ijzerman, A.P., et al. Biological and pharmacological roles of HCA receptors. Adv. Pharmacol. 2011; 62:219-50.
- Boyonoski, A.C., Spronck, J.C., Gallacher, L.M., et al. Niacin deficiency decreases bone marrow poly (ADP-ribose) and the latency of ethylnitrosourea-induced carcinogenesis in rats. J. Nutr. 2002; 132:108-14.
- Cresci, G.A., Thangaraju, M., Mellinger, J.D., et al. Colonic gene expression in conventional and germ-free mice with a focus on the butyrate receptor GPR109A and the butyrate transporter SLC5A8. J. Gastrointest. Surg. 2010; 14:449-61.
- During, A., Penel, G., Hardouin, P. Understanding the local actions of lipids in bone physiology. Prog. Lipid. Res. 2015; 59:26-46.
- Graff, E.C., Norris, O.C., Sandey, M., et al. Characterization of the hydroxycarboxylic acid receptor 2 in cats. Domest. Anim. Endocrinol. 2015; 53:88-94.
- Guntur, A.R., Rosen, C.J. Bone as an endocrine organ. Endocr. Pract. 2012; 18:758-62.
- Hanson, J., Gille, A., Zwykiel, S., et al. Nicotinic acid- and monomethyl fumarate-induced flushing involves GPR109A expressed by keratinocytes and COX-2-dependent prostanoid formation in mice. J. Clin. Invest. 2010; 120:2910-9.
- Hukovic, N., Panetta, R., Kumar, U., et al. Agonist-dependent regulation of cloned human somatostatin receptor types 1-5 (hSSTR1-5): subtype selective internalization or upregulation. Endocrinology. 1996; 137:4046-9.
- Kaczmarek, E., Górna, A., Majewski, P. Techniques of image analysis for quantitative immunohistochemistry. Akad. Med. Bialymst. 2004; 49:155-8.
- Kirkland, J.B. Niacin status and genomic instability in bone marrow cells; mechanisms favoring the progression of leukemogenesis. Subcell. Biochem. 2012; 56:21-36.
- Kostecki, L.M., Thomas, M., Linford, G., et al. Niacin deficiency delays DNA excision repair and increases spontaneous and nitrosourea-induced chromosomal instability in rat bone marrow. Mutat. Res. Fund. Mol. Mech. Mut. 2007; 625,50-61.
- Kostylina, G., Simon, D., Fey, M.F., et al. Neutrophil apoptosis mediated by nicotinic acid receptors (GPR109A). Cell Death Differ. 2008; 15:134-42.
- Parfitt, A.M., Drezner, M.K., Glorieux, F.H., et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. Bone Miner. Res. 1987; 2:595-610.
- Schaub, A., Fütterer, A., Pfeffer, K. PUMA-G, an IFN-gamma-inducible gene in macrophages is a novel member of the seven transmembrane spanning receptor superfamily. Eur. J. Immunol. 2001; 31:3714-25.
- Shomali, T., Tadjalli, M., Akhavan Taheri, R. Niacin exacerbates methyl prednisolone-induced bone changes in growing rats. Iran. J. Pharmacol. Ther. 2013; 12:62-5.
- Shomali, T., Mosleh, N., Kamalpour, M. Screening of different organs of rats for HCA2 receptor mRNA. Int. J. Mol. Cell. Med. 2014; 3:126-9.
- Soga, T., Kamohara, M., Takasaki, J., et al. Molecular identification of nicotinic acid receptor. Biochem. Biophys. Res. Commun. 2003; 303:364-9.
- Spronck, J.C., Kirkland, J.B. Niacin deficiency increases spontaneous and etoposide-induced chromosomal instability in rat bone marrow cells in vivo. Mutat. Res. Fund. Mol. Mech. Mut. 2002; 508: 83-97.
- Thangaraju, M., Cresci, G.A., Liu, K., Ananth, S., et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009; 69:2826-32.
- Thomas, R.F., Holt, B.D., Schwinn, D.A., et al. Long-term agonist exposure induces upregulation of beta 3-adrenergic receptor expression via multiple cAMP response elements. Natl. Acad. Sci. USA. 1992; 89:4490-4.
- Titgemeyer, E.C., Mamedova, L.K., Spivey, K.S., et al. An unusual distribution of the niacin receptor in cattle. Dairy. Sci. 2011; 94:4962-7.
- Torhan, A.S., Cheewatrakoolpong, B., Kwee, L., et al. Cloning and characterization of the hamster and guinea pig nicotinic acid receptors. J. Lipid. Res. 2007; 48:2065-71.
- Tunaru, S., Kero, J., Schaub, A., et al. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat. Med. 2003; 9:352-5.
- Wakade, C., Chong, R. A novel treatment target for Parkinson’s disease. J. Neurol. Sci. 2014; 347:34-8.
- Wise, A., Foord, S.M., Fraser, N.J., et al. Molecular identification of high and low affinity receptors for nicotinic acid. J. Biol.Chem. 2003; 278:9869-74.
- Wonnacott, The paradox of nicotinic acetylcholine receptor upregulation by nicotine. Trends Pharmacol. Sci. 1990; 11:216-9.







