Zeynab zanguei1 and Habibollah jowhary2*
1Department of biology, Jahrom Branch, Islamic Azad University, Jahrom, Iran 2Department of physiology, Darab Branch, Islamic Azad University, Darab, Iran
DOI : https://dx.doi.org/10.13005/bpj/821
Abstract
Cardiovascular diseases and atherosclerosis that develops through gradual fatty deposits in the lower part of vascular endothelium are the main causes of death in the world. This research intended to study the effects of hydroalcoholic extract of valerian on the quantity of lipoproteins and on the development of atherosclerosis in the aortas of male hypercholesterolemic rabbits. This empirical research was carried out on 30 male New Zealand rabbits that were divided into 5 groups each with 6 members. The control group received no medicine. Sham group one were given 10 ml of corn oil (a cholesterol solvent) every day, sham group two 10 ml of cholesterol every day, and the experimental groups one and two 50 and 200 ml of hydroalcoholic extract of valerian, respectively. At the completion of the research, the data was analyzed using ANOVA and Duncan’s test, and Excel was employed to draw the figures. Concentrations of HDL, LDL, and triglycerides in the cholesterol sham group increased significantly compared to the control group, but significantly decreased in the experimental groups compared to the cholesterol sham group. Valerian improves HDL, LDL, and triglyceride levels in male hypercholesterolemic rabbits.
Keywords
Valerian; hypercholesterolemic; atherosclerosis; rabbit
Download this article as:| Copy the following to cite this article: Zanguei Z, Jowhary H. Hydroalcoholic Extract of Valerian, Blood Factors, and Atherosclerotic Plaque Formation in Male Hypercholesterolemic Rabbits. Biomed Pharmacol J 2015;8(2) |
| Copy the following to cite this URL: Zanguei Z, Jowhary H. Hydroalcoholic Extract of Valerian, Blood Factors, and Atherosclerotic Plaque Formation in Male Hypercholesterolemic Rabbits. Biomed Pharmacol J 2015;8(2) .Available from: http://biomedpharmajournal.org/?p=3200> |
Introduction
Atherosclerosis, commonly called hardening of the arteries, is a vascular disease; and it is very important to know and control factors that cause and aggravate it [1]. This disease has always been the most important cause of death in developed countries and accounts for about 50% of deaths [2, 3]. In the United States, coronary heart disease is the main cause of death in men over the age of 40 and women over the age of 65[4]. Atherosclerosis results from lipid deposits in large and medium-sized arteries [5, 6] and causes hardening of artery walls, which is followed by decreased elasticity and narrowing of blood vessels that eventually results in reduced blood supply to important body organs including the heart and the brain [7].
Although increased plasma lipids is a known risk factor for vascular diseases, in about half of patients the levels of these lipids do not change and, hence, the increased risk for coronary heart disease cannot be explained. Therefore, this disease can be better diagnosed in these patients by measuring the apolipoprotein level [8].
At present, this disease can be effectively prevented in people susceptible to it by monitoring various blood biochemical factors. Hyperlipidemia, diabetes mellitus, age, gender, hypertension, and smoking are important risk factors for cardiovascular diseases and cause total and serum cholesterol and LDL-C to rise and HDL-C to fall abnormally [9, 10]. High cholesterol concentration in blood plasma, which is often detected as low-density lipoprotein (LDL), is considered the most important cause of atherosclerosis. Research has shown oxidation of this compound (LDL oxidation) signifies the first stage in the development of atherosclerosis in cardiovascular diseases [11, 12]. LDL oxidation plays an important part in the development of atherosclerosis. Utilization of oxidants in food materials leads to LDL oxidation that causes the development, and progress, of atherosclerosis [13]. When LDL is oxidized, the affinity it has for its receptor decreases. Accumulation of oxidized LDL in macrophages leads to the formation of foam cells and the development of atherosclerosis [14].
Since atherosclerosis is considered an inflammatory disease, inflammation and factors influencing it are potential risk factors [15, 16], and research has indicated acute-phase proteins are useful in predicting the risk for cardiovascular diseases [17].
Controlling these risk factors is important in preventing atherosclerosis, and nowadays use of medicinal plants for controlling the risk factors has attracted interest [18].
In traditional Iranian and Islamic medical sources, utilization of plants including valerian for treating digestive disorders and indigestion together with flatulence and/or constipation attracted interest [19]. Valerian is an herbaceous plant with a strong stem and height of 0.5-2 m growing wildly in sparsely-treed forests [20]. Its roots and rhizomes contain amidon, tannin, glucose, formic acid, acetic acid, and a large quantity of manganese [21]. Valerian roots have strong antispasmodic effects. They were used in traditional medicine to also cure problems of nervous origin, hysteria, as a reducer of urine volume in diabetics, for curing insomnia and expelling stomach gas, and as an antispasmodic and a tranquilizer [22]. Moreover, valerian roots are used to treat nervous disorders, especially dizziness, nerve pain, headache, migraine, anxiety, disorders associated with menopause, continuous hiccups, and stomach pain [23]. In recent research on valerian, its hypnotic, antispasmodic, analgesic, and blood pressure reducer effects have been mentioned [26]. Phytochemical studies have indicated valerian is rich in flavonoids that are mainly in the form of flavanols and have antioxidant properties [21].
The large quantities of valerian used in traditional medicine, and its useful and influential effects and very low toxicity, prompted us to study its effects on the progress of atherosclerosis in male hypercholesterolemic rabbits.
Materials and methods
Preparation of the extract
Valerian plants were dried for 10 days at room temperature and powdered using an electric grinder. One hundred grams of the powder were then soaked in 96% ethanol for 72 hours, filtered, and concentrated using a vacuum distillation apparatus. The concentrated solution was decanted in three stages (once with 100 and twice with 50 ml of chloroform). The solution obtained from the last stage was poured in a container, dried at 50˚C under sterile conditions, and kept in a dark glass bottle at 4˚C [27].
Grouping and treating rabbits
Thirty male New Zealand rabbits each weighing 2000-2500 grams were bought from the Razi Research Institute and kept for two weeks in the animal den at the Islamic Azad University of Jahrom to get acclimated. They were fed the base diet of Super Fosskon Standard Rabbit Chow (which included protein, fiber, and fats at 14, 150, and 30 g/kg, respectively) during these two week [28]. The rabbits were then divided into 5 groups each with 6 members and fed the following diets by gavage for 45 days [29]:
Group 1 (control group) received the ordinary diet
Group 2 (sham group one) were given corn oil as a cholesterol solvent
Group 3 (Sham group two) were fed a high cholesterol (5% cholesterol) diet [28]
Group 4 (Experimental group one) received a high cholesterol diet together with the minimum dose of valerian extract (200 mg/kg)
Group 5 (Experimental group two) were given a high cholesterol diet together with the maximum dose of valerian extract (800 mg/kg). During this period, the rabbits had free access to food and water. To prepare a high cholesterol diet, the cholesterol bought from the German Merck Company constituted one percent of the daily diet.
Measuring biochemical factors
Before administering the diets, and at the completion of the research, the rabbits were weighed. Blood samples were taken from the heart of each rabbit and the sera were used to determine total cholesterol, triglycerides, LDL, and HDL concentrations using biological kits and a Hitachi Model 902 Automatic Analyzer.
Statistical Analysis
Results were analyzed in the form of Mean ± SD. ANOVA and Duncan’s test were employed for studying the biochemical results and to compare the means of the experimental groups using SPSS 17, and Excel was employed for drawing the figures.
Results
Figure 1 shows weights of rabbits in experimental group two significantly decreased compared to those in the control and the sham groups ( p<0.05), but the weights of rabbits in the corn oil and cholesterol sham groups significantly increased compared to those in the control group.
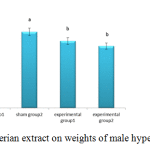 |
Figure 1: Effects of valerian extract on weights of male hypercholesterolemic rabbits |
Figure 2 indicates HDL levels in the groups receiving cholesterol significantly increased (p< 0.05), and HDL levels in experimental group two increased compared to experimental group
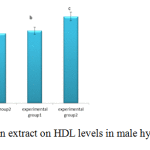 |
Figure 2: Effects of valerian extract on HDL levels in male hypercholesterolemic rabbits |
Figure 3 shows weights of rabbits in the sham group receiving cholesterol significantly increased compared to the control group, but HDL levels in experimental group two (that were given a high cholesterol diet with the maximum dose of valerian extract) decreased significantly ( p< 0.05).
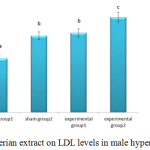 |
Figure 3: Effects of valerian extract on LDL levels in male hypercholesterolemic rabbits |
Figure 4, which presents changes in cholesterol levels in the various groups, shows total cholesterol significantly increased in the group treated with cholesterol ( p<0.05), but cholesterol levels significantly decreased in groups that were given valerian extract together with high cholesterol diets ( p<0.05).
://biomedpharmajournal.org/wp-content/uploads/2015/12/fig-45-150×150.gif” alt=”figure 4″ width=”150″ height=”150″ /> |
Figure 4: Effects of valerian extract on cholesterol levels in male hypercholesterolemic rabbits |
Figure 5, which shows changes in triglycerides levels, indicates triglycerides levels significantly increased in the group receiving cholesterol (p<0.05) but significantly decreased in experimental groups one and two compared to the sham group receiving cholesterol (p<0.05).
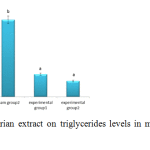 |
Figure 5: Effects of valerian extract on triglycerides levels in male hypercholesterolemic rabbits |
Table 1: Comparison of biochemical factors and weights of rabbits in the studied group
| control | sham group(corn oil) | sham group(cholesterol) | experimental group 1 | experimental group2 | |
| HDL( mg/dl) | 50.8±1.28a | 61.4±1.17a | 87.95±22.23b | 90.73±7.48b | 108.25±4.39c |
| LDL( mg/dl) | 29.8±1.11a | 32.4±1.38a | 132.56±30.28b | 95.73±7.88a | 76.25±4.03a |
| (mg/dl) cholesterol | 57.83±1.47a | 61.13±1.57a | 759.25±80.75b | 215.21±50.43a | 151.87±60.37a |
| triglycerides (mg/dl) | 73.11±2.45a | 82.25±2.89a | 689.75±26.86b | 201.10±49.80a | 141.19±37.56a |
| weight (gr) | 1500.31±256.85b | 1725.98±154.61a | 1850.69±184.47a | 1625.13±115.10b | 1502.05±150.52b |
The symbol * indicates values were presented in the form of Mean ± SEM.
The symbol * shows the statistical level employed was p<0.05.
Discussion
In this research, high cholesterol diets increased total cholesterol, triglycerides, and LDL levels, and use of these diets also significantly reduced HDL concentrations. Similar changes were reported in previous studies [30].
Results concerning the effects of various doses of the hydroalcoholic extract of valerian on lipid levels in blood serum of hypercholesterolemic rabbits are as follows. Total cholesterol, triglycerides, and LDL cholesterol levels of rabbits in the group receiving high cholesterol diets together with various doses of the extract decreased significantly, depending on the extract dose, compared to the group receiving high cholesterol diets, at the completion of the research. Moreover, HDL cholesterol concentrations in this group significantly increased (depending on the extract dose) compared to the group receiving high cholesterol diets. The maximum extract dose was more effective in reducing cholesterol, triglycerides, and LDL levels, and in increasing HDL levels, compared to the minimum dose. These results show the effectiveness of the hydroalcoholic extract of valerian in modifying the dyslipidemia resulting from administering high cholesterol diets to the rabbits.
Previous research found one of the starting stages of atherosclerosis was the entry of low-density lipoproteins into artery walls. This caused accumulation, modification, and oxidation of low-density lipoproteins, which prevented the adhesion, migration, and differentiation of monocytes and caused inflammation [31]. Moreover, researchers stated glycosylation and all events that increased oxidative shocks were important factors [32-35]. Furthermore, previous studies showed that atherosclerosis was an inflammatory process [36]. Inflammation, especially chronic inflammation, is one of the common complications of many diseases and weakens the immune system of the body. The inflammation process, in addition to creating infection problems, delays the healing process in the related diseases [36]. Rhizome roots of valerian contain flavonoids, amidon, tannin, glucose, various salts, essential oil, valerinic acids, formic acid, acetic acid, and propionic acid. Studies on the total plant fractions showed the flavonoids in valerian, due to their special spatial shape, accelerate intestinal absorption, possess significant anti-inflammatory effects, increase suppression of prostaglandins, inhibit 5-lipooxygenase and, thus, normalize concentrations of biochemical factors in blood serum [37].
Moreover, people with hyperlipidemia require more antioxidants and their blood lipids can be reduced by adding antioxidants to their diets or through administering drugs. Phytochemical studies have shown valerian is rich in flavonoids that are mainly in the form of flavanols and possess antioxidant properties [21] and, thus, reduce lipid peroxidation and oxidative destruction of blood vessels.
Conclusions
Results of this research indicate use of valerian extract together with high cholesterol diets reduces triglycerides, cholesterol, HDL, and LDL. This shows the strong anti-inflammatory and antioxidant properties of this extract, which reduced atherosclerotic lesions in the group receiving the extract. Considering the strong effects of this extract in reducing lesions, further research should be carried out on this plant and on its molecular mechanisms.
Acknowledgements
The authors of this article would like to express their gratitude to the Research Deputy of the Islamic Azad University of Jahrom.
Conflict of interests
The authors of this article did not declare any conflict of interest in compiling and publishing this article.
Reference
- Arshadi A, Talebi E, Taheri Y, Kargar H. Studying protective effects of oats and barley extract on some blood parameters (hdl, cholesterol and triglycerides) in rats fed high-fat diet. jjums. 2014; 12 (2) :31-37.
- Gaziano JM. Epidemiology of risk factor reduction. 1nd. Boston: Little Brown.2000; 569-586.
- Lusis, A.J. Atherosclerosis. Nature. 2000;407:237-241.
- Faggiotto A, Ross R, Harker L. Studies of hypercholesterolemia in the nonhuman primate, In : changes that lead to fatt streak formation. Arterioscler.2000; 4 (4): 323–340.
- Campbell JH, Efendy JL, Smith NJ, Campbell GR. Molecular basis by which garlic suppresses atherosclerosis. J Nutr .2001;131(3s):1006-9.
- Madani H, Jafari Dinani N, Mahzoni P, Asgary S. Effect of glycyrrhiza glabra extract on aorta wall atherosclerotic lesion in hypercholesterolemic rabbits.Pak J Nutr.2007;6(4):313-17.
- Ahaneku, J.E., Nwosu, C.M. and Farotimi, A.Lipid and lipoprotein cardiovascular risk factor responses to episodic academic stress. Journal of Health Science.2001; 47, 3: 323-326.
- Rasouli, M., Kiasari. M.A. and Mokhberi, V.The ratio apoB/apoA, apoB and lipoprotein(a) are the best predictors of stable coronary artery disease.Clinical Chemistry and Laboratory Medicine.2006;44(8): 1015-1021.
- Grundy, S.M., Pasternak, R. and Greenland, P. Assessment of cardiovascular risk by use of multiple-risk professionals from the American College of Cardiology. Journal of the American College Cardiology.2000;34: 1348-59.
- Parmley, W.W. Nonlipoprotein risk factors for coronary heart disease: evaluation and management. American Journal of Medicine.2000; 102: 7-14.
- Bahorun T, Soobrattee MA, Luximon-Ramma V, Aruoma OI. Free radicals and antioxidants in cardiovascular health and disease. Internet Journal of Medical Update .2006;1:13-17.
- Beattie J, Crozier A, Garry GD. Potential health benefits of berries. Current Nutrition & Food Science.2005;1:71-86.
- Willcox BJ, Curb JD, Rodriguez BL. Antioxidants in cardiovascular health and disease: key lessons from epidemiologic studies. Am J Cardiol.2008; 101(10A): 75D- 86D.
- Ahmadvand H, Shahsavari G, Abdolahpour F, Bagheri S, Rashidi M. The inhibitory effects of Walnut (Juglansregia L.) huskhydroalcoholic extract on LDL oxidation in vitro. Jjums.2011; 9 (3) :1-7
- Fuster V, Alexander R, Rourke R. Hurst’s the heart.11th edition. New York: Mc Graw-Hill;2005.
- Yasuaki D, Hiroyuki T, Koichi S, Ryuzo U. Association among C-reactive protein, oxidative stress, and traditional risk factors in healthy Japanese subjects. Intern J Cardiology.2007;115: 63-66.
- Thor E, Verônica F. Relationship Between CReactive Protein Levels and Regional Left Ventricular Function in Asymptomatic Individuals: The Multi-Ethnic Study of Atherosclerosis. J of the Am College of Cardiology.2007; 49: 594-600.
- Kasper D, Braunwald E, Fauci A, Hauser S, Lonso D, Lameson J. Haerrison’s principles of inter medicine, 6th edition. New York: Mc Graw-Hill; 2005.
- Jafari H, Gharebaghi R.The effect of Valeriana Officinalis and Amitriptyline on Reserpinated rats. Journal of Qazvin Univ Med Sci. 2001; 5(16):3-6. [Article in Persian]
- Sajjadi SE, Tavakoli N, Samaian M. Formulation of a sedative film coated tablet from extracts of Melissa Officinalis and Valeriana officinalis. Journal of Kerman Univ Med Sci. 2003; 2(10):71-78. [Article in Persian]
- Solati J, Sanaguye Motlagh H. Anxiolytic effects of Valepotriates extracted from Valeriana officinalis L. in rats. Journal of Qazvin Univ Med Sci. 2008; 12(3): 63-67. [Article in Persian]
- Karimi GH, Hossainzadeh H, Bakhtyari H. Study of anticonvulsant activity of valeriana Officinalis roots and rhizomes hydroalcoholic extract in mice and relation to nitric oxide.Journal of Medicinal Plants. 2003; 2(7):43-48 [Article in Persian]
- Fields AM, Richards TA, Felton JA, Felton SK, Bayer EZ, Ibrahim IN, et al. Analysis of responses to valerian root extract in the feline pulmonary vascular bed. J Altern Complement Med. 2003;9(6):909-18.
- Heidari MR, Razban F. Effects of Valeriana Officinalis extract on the seizure induced by Picrotoxin in mice. Journal of Kerman University of Medical Sciences. 2004; 2(11):100-108. [Article in Persian]
- Vohora SB, Dandiya PC. Herbal analgesic drugs. Fitoterapia. 2002; 63(2): 95-107.
- Gilani AH, Khan AU, Jabeen Q, Subhan F, Ghafar R. Antispasmodic and blood pressure lowering effects of Valeriana wallichii are mediated through K+ channel activation. J Ethnopharmacol. 2005 ; 14;100(3):347-52.
- Eseyin, O.A., Ebong, P., Ekpo, A., Igboasoiyi, A. and Oforah, E. Hypoglycemic effect of the extract of Valeriana Officinalis in rat. Pakistan Journal of Biological Sciences.2007;10(3): 498-501.
- Prasad K. Reduction of serum cholesterol and hypercholesterolemic atherosclerosis in rabbits by secoisolariciresinol diglucoside isolated from flaxseed. Circulation.2000; 99: 1355-1362.
- Mirhydar H. Preventation and treatment of diseases with herbal medicin. 5th Publication office Islamic culture. Tehran. 2001; p: 527.
- Flora G, BakerA.B, Loewenson R.B, Klassen A. A Comparative Study of Cerebral Atherosclerosis in Males and Females. Circulation.2001. 38, 859-869.
- David LN, Micheal MC. Lehninger principle of biochemistry .Phiadelphia:Sunders company.2000;770-816.
- Katsuyuki N, Takamitsu N, Akira T. The oxidative modification hypothesis of artherosclolerosis:the comparison of atherogenic effects on oxidized LDL and remnant lipoproteins in plasma. Clinica chimioca Acta.2006;367:36-47.
- Clermont P, Creager M, Losodo D, Gregory K, Dzau V. Atherosclerosis:recent discover s and novel hypoyheses. Circulation.2005;112:3348-3353.
- Braunwald ZS. Libby heart disease . atext book of cardiovascular medicine.7th ed .2005;ch.31:1010.
- Maurizio N. Plasma levels of oxidized-low-density lipoproteins are higher in patients with unstable angina and correlated with angiographic coronary complex plaques.Atherosclerosis.2006;185:114-120.
- Faggiotto A, Ross R, Harker L. Studies of hypercholesterolemia in the nonhuman primate , In:changes that lead of fatty streak formation.Artriosclerosis.2004;4:323-340.
- Shahidi F and Naczk M. Phenolics in food and nutraceuticals (Boca Raton, Florida, USA: CRC Press,).2004; pp. 313–314 ISBN 1-58716-138-9.








