Manuscript accepted on :
Published online on: 25-12-2015
Plagiarism Check: Yes
S. Parthiban1, T. Ramakrishnan2, V. Shankarram3, V. Balakumar4, Rathika rai5, Umasudhakar6
1Senior lecturer, Department of periodontics., Thai moogambigai dental college, Golden George nagar, Chennai 600107 2Professor, Department of periodontics.,Adhi parasakthi dental college, Melmaruvathur, Tamil nadu 3Professor, Department of Periodontics., Thai moogambigai dental college, Golden George nagar, Chennai 600107 4Professor, Department of prosthodontics., Satyabama dental college, Chennai 5Professor, Department of prosthodontics., Thai moogambigai dental college, Golden George nagar,Chennai 600107 6Professor, Department of Periodontics., Thai moogambigai dental college, Golden George nagar, Chennai 600107.
DOI : https://dx.doi.org/10.13005/bpj/810
Abstract
1) To compare qualitative and quantitative profile of herpes viruses In gingival cervicular fluid of periodontitis and healthy periodontal tissues ; 2) To quantitatively assess the relation between HSV and periodontitis 40 patients with periodontitis(P) ,40 patients with gingivitis (G)and 40 with Healthy gingival tissues (HP) were recruited. GCF samples from periodontal sulcus were analyzed using quantitative real-time PCR to detect and quantify HSV virus. Frequencies of detection and levels of HSV were compared between healthy and diseased. Correlation between HSV viruses and Clinical parameters was assessed. Among the HSV viruses ,EBV prevalence was slightly higher in periodontitis sites compared with controls (33.3% vs. 23.8%; p>0.05); HSV-1 and HCMV viruses were less dominant.Significant correlation was seen between the HSV and periodontitis clinical parameters. Prevelance of HSV viruses in GCF and its correlation between the clinical parameters may represent an unspecific indicator for the host response in periodontitis.
Keywords
HSV viruses; GCF; Periodontitis
Download this article as:| Copy the following to cite this article: Parthiban S, Ramakrishnan T, Shankarram V, Balakumar V, Rai R, Umasudhakar. Estimation of Load of Herpes Viruses in GCF and its Impact on Clinical Parameters in Periodontitis. Biomed Pharmacol J 2015;8(2) |
| Copy the following to cite this URL: Parthiban S, Ramakrishnan T, Shankarram V, Balakumar V, Rai R, Umasudhakar. Estimation of Load of Herpes Viruses in GCF and its Impact on Clinical Parameters in Periodontitis. Biomed Pharmacol J 2015;8(2).Available from: http://biomedpharmajournal.org/?p=3142> |
Introduction
Periodontal diseases are usually a chronic inflammatory and infectious disease caused primarily by microorganisms leading to attachment loss and tooth loss (1) plaque and calculus are considered to be the primary etiology of disease although host response is considered equally important in progression of disease,resulting in destruction of the structural components (2)
Although Gram negative bacteria are usually isolated in the area of periodontal destruction,bacterial etiology is not alone considered in causing destruction as rapid periodontal destruction some times occur in the area of minimal local factors or low level risk factors(3), refractory periodontitis occur after a succesfull periodontal therapy which involves removal of supragingival and subgingival local factors(4) specifity at certain sites(5) varied distribution of disease(6) progression from gingival periodontal disease (7) .so some other factors which play a role in degradation of tissues has to be revealed.
Viruses particularly herpes viruses and their biology may provide some answers for better understanding of mechanisms involved in the degradation of periodontal tissues. As Herpes viruses are detected in diseased sites and in gingival crevicular fluid in mild (8) and severe (9) and aggressive [10] periodontitis as well as in the periodontium of HIV patients [11] and patients with the followingsyndromes: Papillon-Lefévre [12], Down [13] and Kostmann [14].
Thus the etiology of periodontitis is considered complex and multifactorial which involves variety of microorganisms ( bacteria,viruses,fungi ),host factors ,immunological responses.
Objective
The aim of this study was to investigate the prevalence of herpes viruses in gingival crevicular fluid of healthy and diseased periodontium in indianpopulation and to explore whether there is a correlation between the load of this virus in GCF and the level of periodontal destruction.
Methods
The study, approved by the Ethical Committee of the Dr.MGR University and research institute ,. The group of patients with periodontitis (periodontitis group -PG) consisted of 40 patients (20 men and 20 women, age 23-76) who had clinical signs of periodontitis and were treated at the department for Periodontology and. Healthy control group (HC) consisted of 40 volunteers(20 men and 20women, age 18-33) without clinical signs of periodontitis.
Exclusion criteria for the study were:1) known systemic diseases (cardiovascular, respiratory, renal,malignancy, etc.), 2) presence or history of any severeinfections, 3) systemic antibiotic or immunomodulatory treatment in the previous 3 months, 4) long-term treatmentwith any medication suspected to affect the periodontium(e.g. non-steroidal anti-inflammatory drugs), 5) pregnant or lactating women and 6) less than 20 teeth present, 7) less than 3 teeth from Ramfjord examination model, (8) any therapy of periodontitis 1.5 year period prior to the study.
Clinical Outcome Variables and radiographic examination
A full mouth periodontal examination was performed in all patients recording the following clinical parameters at six sites using a periodontal probe graded in mm*:
1) Clinical PD in mm,
2) BOP measured 15 seconds after probing and recorded as present (1) or absent (0), 14
3) Visible plaque accumulation (PI) measured along the mucosal margin and recorded as present (1) or absent (0). 15 These measurements were performed by two examiners (L.C., P.P.) after a calibration exercise demonstrating 95.7% concordance within ± 1 mm for measurements. Radiographs were taken from all the diseased sites using a paralleling technique combined with long cone.
Microbiological sampling
The supragingival plaque was removed by sterile cotton pellets micropipettes is inserted in gingival sulcus/periodontal pocket until a mild resistancefor 10 minutes. GCF contaminated with blood were not used in the analysis. Gcf was collected in small plastic tubes samples were stored at-70°C until further analysis.
Quantitative real-time PCR assays for Epstein Barr Virus, HSV-1, HCMV
The PCR procedure was carried out at the Laboratory for Molecular Biology,(hitech laboratories Chennai). Quantitative real-time PCR was performed to detect the presence/absence and quantify the Epstein-Barr virus (EBV) Herpes simplex virus-1(HSV-1)& Human cytomegalo virus(HCMV). First, the total viral DNA was isolated using reagents according to the manufacturer’s guidelines**. Then, real-time PCR was carried out for HSV using the Herpesvirus quantitative Real Time PCR kit†† and a thermal cycling system‡‡. Briefly, quantitative real-time PCR assays were performed in a volume of 25 Wl composed of 12,5 Wl of DNA mastermix 2,5Wl of primers***(FAM, emission 520nm), 2,5 Wl of internal control DNA†††, 2,5 Wl of internal control primers & double-dye probe (Yellow Dye, emission 548 nm)‡‡‡and 5 Wl of DNA extract or EBV positive control or EBV negative control or DNAStandard (for quantitative standard curve)§§§. Five EBV DNA dilutions were used for the standard curve (from 200 copies to 2.000.000 copies of HSV amplicon/PCR reaction).
Statistical analysis
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) for Windows, version 15.. The Independent SamplesT-test was used for comparing continuous variables. Statisticaldifferences between frequencies were tested with Chi-square test with Yates’ correction. In all analyses the significance level was set at 0.05.
After training and calibration, the samples were collected. For the evaluation of intra- and inter- reliability, 10% of randomly selectedsubjects were re-examined 2 weeks after the first examination.
Results
The Kappa scores were 0.5-0.7, representing a very good agreement [20].The age and sex distribution of study subjects are shown in Table 1. HC and PG subjects were matched. Clinical parameters for both groups arepresented in Table 2.There were no statistically significant differences in the presence of HSV-1,HCMV,EBV between gingivitis(38.9%) and HP (32.3%) groups (Chi-square test, with Yates’ correction p=0.7574).
The difference in mean age was not found neither between the patients with or without HSV-1 nor between genders. Sixteen female (38.1%) and 8 male (32.0%) patients were positive for HSV-1,EBV,HCMV in gingival crevicular fluid samples(Chi-square test with Yates’ correction, p=0.8105). Furthermore,the distribution of HSV-1 (Chi-square test withYates’ correction, p=0.938). We also tried to establish acorrelation between the presence of HSV-1 ,EBV,HCMV and the recurrence of oral herpes infections. Seventeen patients couldnot recall if they ever had a recurrent herpes infection. For the rest of them, 14 patients noted at least one episode of herpes labialis, and 36 denied it. There was no statistically significant difference in the presence of HSV-1 betweenpatients who had recurrent HSV infection in the oral region (50.0% were HSV positive) and patients who had not(38.9% were HSV positive) (Chi-square test, p=0.6924).
However, within the PG group, several clinical parametersdiffered significantly depending on the presence ofthe virus (Table 3). Probing pocket depth and clinical attachment loss revealed higher values in the patients with HSV, while plaque index was lower in the HSV positive group than in the HSV-1 negative group. In addition, HSV occurred more often in deeper pockets (Table 4),and three out of four deepest pockets measuring 11 mm harbored HSV1.
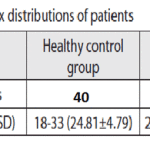 |
Table 1: Age and sex distribution of patients |
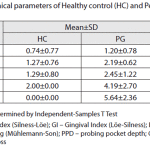 |
Table 2: Clinical pararmeters of healthy control and periodontitis group |
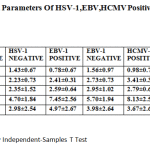 |
Table 3: Clinical Parameters of HSV-1,EBV,HCMV Positive and Negative Patients |
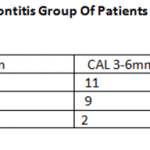 |
Table 4: Herpes Viruses in Periodontitis Group of Patients According to the Pocket Depth and CAL |
Discussion
As previously mentioned, the etiopathogenesis of the periodontal disease is not completely clarified. The initial event in the development of periodontitis is the formation of dental biofilm followed by gingivitis. T lymphocytes, Blymphocytes and monocytes/macrophages infiltrate can lead to the accumulation of herpes viruses in the periodontaltissue, as these cells are considered to be the source of viruses [21, 22]. Reactivation of herpes viruses can decrease the local host resistance and lead to the overgrowthof periodontal pathogenic bacteria, as Porphyromonas gingivalis[7].
Herpes viruses may contribute to the progression of periodontitis through a number of mechanisms. It is assumed that these viruses are able to express cytopathogenic effects, immune evasion, immunopathogenicity, latency, reactivation and tissue tropism [23]. They can infect or alter structural cells and host defense cells in the periodontium, and thereby reduce the ability of periodontal tissuesto resist bacterial insults [22].
In the multitude of studies dealing with the presence of viruses from the Herpesviridae family in the periodontium,the majority focused on EBV-1 and HCMV [14, 24, 25, 26]. HSV was investigated to a lesser extent [8, 27, 28].
Our results showed a high prevalence of HSV-1 in GCF (35.8%), EBV (25%)HCMV (12%) which is in agreement with some other authors who reported a high prevalence of this virus in specimens taken with paper points plaque samples of periodontalpockets [8, 9, 27]. Contrary to our results, Nibaliet al. [28] found a low prevalence of all investigated herpesviruses, especially HSV-1 in both patients with periodontitis and healthy controls.
In the present study the hypothesis that the presence ofHSV-1,EBV,HCMV is in correlation with the development of periodontitis could not be confirmed because we did not detect any difference in the presence of this virus between the control group and patients with chronic periodontitis. Although,the subjects with a healthy periodontium were much younger than those with periodontitis, this discrepancy should not have an impact on our results as the peak of the primary herpetic infection occurs until the age of five [29].However, we can assume that periodontal disease did not evelop in younger individuals, or did not lead to clinically noticeable tissue destruction yet. Consequently, it would be valuable to conduct a follow-up of young patients with HSV-1 detected in their periodontium and periodically make clinical examinations.As for the different number of male and female individuals in the healthy control group, gender itself is not considered as the predilection factor for periodontal destruction[30]. On the other hand, lactation, pregnancy,oral contraceptives, menstrual period may have an impact on periodontal tissues, which is why we excluded females with any of the mentioned conditions. Regarding the influence of gender on HSV-1 prevalence, no gender differences were found in the study performed in Romania from 2004-2005 [31]. As the prevalence of HSV-1 infection varies among different geographic regions, and Romania borders with Serbia, we consider findings of this study relevant in regard to our population.Contreras and Slots [27] also failed to detect differences in the presence of HSV-1 between PG and HC groups.
On the other hand, Grenier et al. [8] reported a higher prevalence of HSV-1 in subjects with periodontitis than in healthy controls compared to EBV,HCMV. Parra and Slots [9] also found statistically higher prevalence of HSV-1 in patients with chronic periodontitis than in patients with mild gingivitis. The same results were reached by Contreras et al. [22] in gingival tissue specimens. Surprisingly, Bilichodmath et al. [32] found higher prevalence of HSV-1 in patients with chronic periodontitis than in patients with the aggressive form of the disease, but they explained the results as the influence of their patients’ age.
The most important result in our study is the relationship between the presence of HSV-1 ,EBV,HCMVand pocket depth. Our results showed a significantly higher prevalence of HSV-1 in deeper pockets than in shallower ones; clinical parameters (CAL, PPD) also showed significantly higher values in HSV+ periodontitis patients than in HSV-, which is in agreement with the results of Slots et al. [7]. Other authors did not find correlation between the depth of periodontal pockets and HSV-1,EBV,HCMV presence. [8]. Our results also showed lower values for the plaque index in PG HSV+ patients, which speaks in favor of HSV-1 influence on periodontaltissue destruction and confirms the hypothesis that viruses might have influence on periodontitis progression in patients with good oral hygiene [3]. Kamma et al.[33] detected significantly higher frequencies of HCMV, EBV-1 and HSV in active and progressive periodontitis sites than in stable sites.
Herpes viruses and in particular HSV-1 are considered to have a potential role in the pathogenesis of some oral diseases. There is evidence of a higher presence of HCMV, EBV-1 and HSV in Nigerian malnourished children with acute necrotizing ulcerative gingivitis (ANUG) [34]. The hypothesis is that herpes viruses can affect the host’s immune system, facilitating the development of secondary bacterial infections. Sabeti et al. [35] found a clear relationship between symptomatic periapical lesions and the presence of HCMV and EBV. They presume that viral infections contribute to immune impairment, which in turn creates afertile ground for endodontopathogenic bacterial infections.
This model of pathogenesis could be potentially applied to the shifting of gingivitis toward periodontitis. Furthermore, Phases of remission and reactivation of periodontitis might
coincide with the latency and the reactivation of viruses[36], whilst viral tissue tropism could explain the site-specificity periodontal destruction in some patients [37].
Conclusion
In the present study, we demonstrated that the presence of HSV-1 ,EBV,HCMV in the GCF is related to the degree of tissue destruction in the patients with periodontitis. The confirmation of the role of HSV-1,EBV,CMV in the pathogenesis of periodontitis will require a larger sample along with a prospective study that would detect the presence of HSV in the periodontium before the onset, at the time of periodontitisinitiation, and periodically during its development. Also, future studies demonstrating the role of HSV infection in the pathogenesis of periodontitis should prove that eradication of viral infection can prevent the progression of periodontal destruction.
References
- Heitz-Mayfield LJ. Disease progression: identification of high-risk groups and individuals for periodontitis. J Clin Periodontol. 2005; 32(Suppl 6):196-209.
- Pihlstrom BL, Michalowitz BS, Johnson NW. Periodontal diseases. Lancet. 2005; 366(9499):1809-20.
- Cho C-M, You H-K, Jeong S-N. The clinical assessment of aggressive periodontitis patients. J Periodontal Implant Sci. 2011;41(3):143-8.
- Goodson JM, Tanner ACR, Haffajee AD, Sornberger GC, Socransky SS. Patterns of progression and regression of advanced destructive periodontal disease. J Clin Periodontol. 1982; 9(6):472-81.
- Heitz-Mayfield LJ, Schätzle M, Löe H, Bürgin W, Ånerud Å, Boysen H, et al. Clinical course of chronic periodontitis: incidence, characteristics and time of occurrence of the initial periodontallesion. J Clin Periodontol. 2003; 30(10):902-8.
- Papapanou PN. Periodontal diseases: epidemiology. Ann Periodontol. 1996; 1(1):1-36.
- Slots J, Kamma JJ, Sugar C. The herpesvirus-Porphyromonas gingivalis-periodontitis axis. J Periodont Res. 2003; 38:318-23.
- Grenier G, Gagnon G, Grenier D. Detection of herpetic viruses in gingival crevicular fluid of patients suffering from periodontaldiseases: prevalence and effect of treatment. Oral MicrobiolImmunol. 2009; 24:506-9.
- Parra B, Slots J. Detection of human viruses in periodontal pockets using polymerase chain reaction. Oral Microbiol Immunol. 1996; 11(5):289-93.
- Saygun I, Kubar A, Özdemir A, Yapar M, Slots J. Herpesviral-bacterial interrelationship in aggressive periodontitis. J Periodont Res. 2004;39(4):207-12.
- Mardirossian A, Contreras A, Navazesh M, Nowzari H, Slots J. Herpesviruses 6, 7 and 8 in HIV- and non-HIV-associated periodontitis. JPeriodont Res. 2000; 35(5):278-84.
- Velazco CH, Coelho C, Salazar F, Contreras A, Slots J, PachecoJJ. Microbiological features of Papillon-Lefévre syndromeperiodontitis. J Clin Periodontol. 1999; 26(9):622-7.
- Hanookai D, Nowzari H, Contreras A, Morrison JL, Slot J. Herpesviruses and periodontopathic bacteria in trisomy 21periodontitis. J Periodontol. 2000; 71(3):376-84.
- Yildirim S, Yapar M, Kubar A. Detection and quantification ofherpesviruses in Kostmann syndrome periodontitis using real-timepolymerase chain reaction: a case report. Oral Microbiol Immunol.2006; 21(2):73-8.
- Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967; 38(6):610-6.
- Silness J, Löe H. Periodontal disease in pregnanacy II. Correlationbetween oral hygiene and periodontal condition. Acta OdontolScand. 1964; 22(1):121-35.
- Mühlemann HR, Son S. Gingival sulcus bleeding – a leadingsymptom in initial gingivitis. Helv Odontol Acta. 1971; 15(2):107-13.
- Ramfjord SP. The Periodontal Disease Index (PDI). J Periodontol.1967; 38(6):602-10.
- Zelić O. Osnovi kliničke parodontologije. 3rd ed. Beograd: Službeniglasnik; 2006.
- Landis JR, Koch GG. The measurement of observer agreement forcategorical data. Biometrics. 1977; 33(1):159-74.
- Slots J, Contreras A. Herpesviruses: a unifying causative factor in periodontitis? Oral Microbiol Immunol. 2000; 15(5):277-80.
- Contreras A, Nowzari H, Slots J. Herpesviruses in periodontal pocket and gingival tissue specimens. Oral Microbiol Immunol. 2000; 15(1):15-8.
- Slots J. Herpesviruses in periodontal diseases. Periodontol 2000. 2005; 38(1):33-62.
- Klemenc P, Skalerič U, Artnik B, Nograšek P, Marin J. Prevalence of some herpesviruses in gingival crevicular fluid. J Clin Vir. 2005; 34(2):147-52.
- Sunde PT, Olsen I, Enersen M, Beiske K, Grinde B. Human cytomegalovirus and Epstein-Barr virus in apical and marginal periodontitis: a role in pathology? J Med Virol. 2008; 80(6):1007-11.
- Chalabi M, Rezaie F, Moghim S, Mogharehabed A, Rezaei M, Mehraban B. Periodontopathic bacteria and herpesviruses in chronic periodontitis. Oral Microbiol Immunol. 2010; 25(3):236-40.
- Contreras A, Slots J. Typing of herpes simplex virus from human periodontium. Oral Microbiol Immunol. 2001; 16(1):63-4.
- Nibali L, Atkinson C, Griffiths P, Darbar U, Rakmanee T, Suvan J, et al. Low prevalence of subgingival viruses in periodontitis patients. J Clin Periodontol. 2009; 36(11):928-32.
- Scully C. Oral and Maxillofacial Medicine. 2nd ed. London: Churchill Livingstone Elsevier; 2008. p.233-240.
- Chambrone L, Chambrone D, Lima LA, Chambrone LA. Predictors of tooth loss during long-term periodontal maintenance: a systematic review of observational studies. J Clin Periodontol. 2010; 37(7):675-84.
- Arama V, Cercel AS, Vladareanu R, Mihai C, Mihailescu R, Rankin J, et al. Type-specific herpes simplex virus-1 and herpes simplex virus-2 seroprevalence in Romania: comparison of prevalence andrisk factors in women and men. Int J Infect Dis. 2010; 14(Suppl 3):e25-31.
- Bilichodmath S, Mangalekar SB, Sharma DCG, Prabhakar AK, Reedy SB, Kalburgi NB, et al. Herpesviruses in chronic and aggressive periodontitis patients in an Indian population. J Oral Sci. 2009;51(1):79-86.
- Kamma JJ, Contreras A, Slots J. Herpes viruses and periodontopathic bacteria in early-onset periodontitis. J Clin Periodontol. 2001; 28(9):879-85.
- Contreras A, Falkler WA, Enwonwu CO, Idigbe EO, Savage KO, Afolabi MB, et al. Human Herpesviridae in acute necrotizing ulcerative gingivitis in children in Nigeria. Oral Microbiol Immunol.1997; 12(5):259-65.
- Sabeti M, Simon JH, Slots J. Cytomegalovirus and Epstein-Barr virus are associated with symptomatic periapical pathosis. Oral Microbiol Immunol. 2003; 18(5):327-8.
- Kamma JJ, Slots J. Herpesviral-bacterial interactions in aggressive periodontitis. J Clin Periodontol. 2003; 30(5):420-6.
- Şahin S, Saygun I, Kubar A, Slots J. Periodontitis lesions are main source of salivary cytomegalovirus. Oral Microbiol Immunol. 2009; 24(4):340-2..







