Manuscript accepted on :
Published online on: 17-11-2015
Plagiarism Check: Yes
EM Sutrisna1 and Tanti Azizah Sujono2
1Department of Pharmacology of Faculty of medicine and Faculty of pharmacy of Universitas Muhammadiyah Surakarta 2Department of Pharmacy of Faculty of Pharmacy of Universitas Muhammadiyah Surakarta Corresponding Author Email : Em.Sutrisna@ums.ac.id
DOI : https://dx.doi.org/10.13005/bpj/580
Abstract
The aim of this study was to evaluate the combination of 70% ethanol extract of Averrhoa bilimbi L. (A bilimbi L.) fruit & Catharanthus roseus G. (C. roseus G) leaves extracts in lowering blood glucose levels. Twenty-five male Wistar rats were divided into 5 groups. All rats were weighed on day 0, then induced by alloxan 150 mg/KgBW (ip). Group I was treated with 0.126 mg/200 gBW glibenclamide (po); Group II was treated by CMC Na 2 ml/200 gBW po; Group III; IV; and V were treated by the extract combination of A. bilimbi L and C. roseus G 40:40; 40:80; and 80:40 mg/200 gBW respectively, for 15 consecutive days (po). The results showed that the combination A. bilimbi L and C. roseus G doses 40:80 and 80:40 mg/200 gBW had the effect of lowering blood glucose levels in 7 days, but couldn’t prevent renal damage by induction of alloxan.
Keywords
Belimbing wuluh (Averrhoa bilimbi L.); Tapak dara (Catharanthus roseus G.); candidate hypoglycemic
Download this article as:| Copy the following to cite this article: Sutrisna E. M, Sujono T. A. The Combination of Belimbing Wuluh Fruit (Averrhoa Bilimbi L.) and Leaves of Tapak Dara (Catharanthus Roseus G.) From Indonesia as a Candidate Hypoglycemic Agents and Thin Layer Chromatography Profiles. Biomed Pharmacol J 2015;8(1) |
| Copy the following to cite this URL: Sutrisna EM, Sujono T. A. The Combination of Belimbing Wuluh Fruit (Averrhoa Bilimbi L.) and Leaves of Tapak Dara (Catharanthus Roseus G.) From Indonesia as a Candidate Hypoglycemic Agents and Thin Layer Chromatography Profiles. Biomed Pharmacol J 2015;8(1). Available from: http://biomedpharmajournal.org/?p=802 |
Introduction
Diabetes mellitus is defined as a group metabolic disease by hyperglycemia, resulting in a defective secretion of insulin, action of insulin, or both1. Diabetes is one of the most common endocrine diseases found in Indonesia. By the year 2030, diabetes prevalence is estimated at 8 million Indonesians, the world’s fourth largest affected population after India, China, and the U.S.2
There are many Indonesian native plants, which are used empirically to treat diabetes. These crops include bawang umbi, bawang prei, bawang pule, bawang putih, tapakdara, dandanggula, jentik manis, salam, mindi, cincin hitam, bidara upas, pare, mengkudu, lampes, kumis kucing, petai kulit, ceplukan, jengkol, urat, bidara laut, mahoni, duwet, brotowali, seledri, and jambu biji.3
Averrhoa bilimbi L. (A. bilimbi L.), commonly known as belimbing wuluh in Indonesia, belongs to the oxalidaceae family.4 The various extracts of fruit and leaves have many uses, including as antidiabetics5, hepatoprotective6 and as an antithrombotic and antioxidant.7 Catharanthus roseus G.(C. roseus G) belongs to the Apocynaceae family.8 Many researchers state that C. roseus G is effective for treatment as antidiabetics9,10,11,12,13,14 anticancer,15,16,17, antispermatogenic18, antihypertensive19, and antifungal,20,21,22.
In our previous study, A. bilimbi L and leaves of Tapak dara were effective in lowering the blood glucose level in male rats induced by alloxan. The 70% of ethanolic extract of belimbing wuluh fruit, with doses of 20 mg/200 gBW , 40 mg/200 gBW, and 80 mg/200 gBW were able to lower blood glucose levels by a percentage of reduction of blood glucose level of 42.7, 43.3 and 58.95%, respectively. The 70 % ethanolic extract of tapak dara leaves (C. roseus G.), dose of 20 mg/200 gBW, 40 mg/200 gBW, 80 mg/200 g BW were able to lower blood glucose levels by 43.56%, 53.7%, and 58.8% respectively.5.
Based on the potential hypoglycemic effects of the plant, researchers tested the combination of both extracts in lowering blood glucose levels on male rats that were induced by alloxan. This method refers to previous studies by other researchers.23,24,25,26,27,28,29,30,31
Materials and Methods
Plant Materials
A. bilimbi L fruit and tapak dara extract were collected from Boyolali, central Java, Indonesia, and stored in a laboratory of pharmacology of Faculty of Medicine at Muhammadiyah University of Surakarta (March 2013).
Experimental animal
Healthy Wistar male rats aged 2-3 months, weighing 150-250 g. All rats were housed in aluminum cages, placed in a room with a temperature of 28-30,50C and fairly light. The animals were acclimatized for 10 days before the experiment. The study was approved by Ethics Committee, Moewardi Hospital of Surakarta with no 35/1/HREC/2014.
Preparation of extract
Ethanolic extract of A. bilimbi L. fruit & Catharanthus roseus G. leaves were prepared by cold maceration. Then extracts were dried with a rotary evaporator.
Induction of Diabetes
After overnight fasting, all rats were made diabetic by an injection of fresh solution of 150 mg per kg of alloxan monohydrate intra peritoneal. Blood glucose levels were measured on days 0, 5, 7, 9, 13, and 19.
Experimental Design
Rats were randomly placed into 5 groups, with each group consisting of 5 white rats that consisted of a negative control group (-) and a positive control group (+). Groups III, IV, and V were given a combination of extract I, II, and III, respectively. All groups were induced by alloxan 150 mg / kg BW given intraperitoneal (IP). This dose was conducted by other researchers (Dhanabal et al., 2008; Kumar et al., 2010 & Viswanathaswamy et al., 2011). All rats were fed pellets at 20 g/day. On day 5, the blood glucose levels of all rats were measured. On days 5 through 19, the negative control group (-) was treated by aquadest, and the positive control group (+) was treated by glibenclamide 0.126 mg/200g BW peroral.
Group III was treated with a combination of ethanolic extract of A. bilimbi L. fruit 40 mg/200 gBW and C. roseus G. leaves 40 mg/200 g BW. Group IV was treated with a combination of ethanolic extract of A bilimbi L. fruit 40 mg/200 gBW and C. roseus G. leaves at 80 mg/200 g BW. Group V was treated with a combination of ethanolic extract of A. bilimbi L. fruit at 80 mg/200 gBW and C. roseus G. leaves at 40 mg/200 g BW. All treatments were done for 15 consecutive days. All groups were given 40 % of glucose solution every day. Blood glucose levels were measured on days 0, 5, 7, 9, 13, and 19. Blood Urea Nitrogen and creatinine levels were measured on days 0 and 19.
Method of Blood Collection
A total of 0.5 ml blood samples were taken from the rats’ tail vein. It was collected in Eppendorf. Blood glucose, Blood Urea Nitrogen and creatinine were estimated by DiaSys kit reagent.
Method of Thin Layer Chromatography
Analysis of chemical constituents in the ethanolic extract of A. bilimbi L fruit and C. roseus G were conducted using thin-layer chromatography. TLC for C. roseus G was done by silica gel GF254 plate and mobile phase chloroform:Methanol (9.5: 0.5), was then examined by UV254 and UV366. TLC for A. bilimbi L was done using silica gel GF254 plate and mobile phase toluene: ethyl acetate: methanol: glacial acetic acid (7.5: 1.5: 0.8: 0.2) respectively, then was examined by UV254 and UV366.
Statistical analysis
The values of non-specific parameters extract are expressed as percentages. Blood glucose level, Blood Urea Nitrogen and creatinine are expressed as mean±SD and were analyzed using one way anova followed by LSD test with p.0.05 significance.
Result
The Effect of Lowering Blood Glucose Levels
Measurement of blood glucose levels was done on days 0, 5, 7, 9, 13, and 19, after being induced by alloxan 150 mg/kgBW. Blood glucose measurement results are shown in Table 1.
Table 1: Mean and standard deviation of blood glucose levels before and after treatment (n=5)
| Groups | Blood Glucose (mg/dl) | |||||
| Day 0 | Day 5 | Day 7 | Day 9 | Day 13 | Day 19 | |
| Positive control | 84.25 ±8.0 | 199 ±24.5 | 180.5 ±49.4 | 158.3 ±35.6 | 124.8 ±26.3 | 139.5 ±14.2 |
| Negative control | 79 ±3.2 | 181.7±55.3 | 203.75±23.8 | 237.75±32.4 | 189.25±27.4 | 221.25±21.5 |
| Combination I | 87 ±10.7 | 221 ±45.3 | 193.2 ±72.3 | 134.6 ±61.9 | 111.6 ±48.7 | 77.6 ±10.9 |
| Combination II | 97.2 ±11.9 | 262.2±35 | 194.8 ±74.2 | 186.6 ±79.6 | 153.8 ±67.9 | 67.2 ±13.4 |
| Combination III | 77.6 ±21.1 | 278.6±10.6 | 212.2 ±74.5 | 204.8 ±70 | 151.2 ±66.0 | 162.8 ±39.2 |
Positive control: glibenclamide 0.126 mg/200 gBW
Negative control: CMCNa 2 ml/200 gBW
Combination I: Rats treated by combination Blimbing wuluh 40 mg/200 gBW and tapak dara 40 mg/200 gBW
Combination II: Rats treated by combination Blimbing wuluh 40 mg/200 gBW and tapak dara 80 mg/200 gBW
Combination III: Rats treated by combination Blimbing wuluh 80 mg/200 gBW and tapak dara 40 mg/200 gBW
Table 1 indicates that the induction of alloxan 150 mg/kgBW intraperitoneal could increase in blood glucose levels on day 5. Table 3 illustrates that administration of a combination of Blimbing wuluh fruit and tapak dara extract could lower blood glucose levels starting on day 2 until day 15 after treatment of extract.
The statistical analysis by one-way ANOVA showed that the positive control (glibenclamide 0.126 mg/200 gBW) were able to lower blood glucose levels, a result significantly different from the negative control both on the second day until after 15 days of treatment, which would indicate that method of test is valid. After two days of extract, the ANOVA test showed that the second and third doses of the combination decreased blood glucose levels significantly, being 4 days to 15 days after administration of the extract either a combination of I, II and III could lower blood glucose levels significantly (p < 0.05). A single dose of alloxan was able to increase the levels of BUN and creatinine, indicating that alloxan is nephrotoxic . BUN and creatinine data are shown in Table 2 and 3.
Table 2: Mean and standard deviation of Blood Urea Nitrogen (BUN) levels before and after treatment (n=5)
| Groups | BUN (mg/dl) | |
| Day 0 | Day 19 | |
| Positive control | 21.13±1.3 | 37.94 ±7.6 |
| Negative control | 22.88±2.4 | 32.57±5.5 |
| Combination I | 21.16±2.0 | 38.94±14.5 |
| Combination II | 22.51±2.2 | 28.30±12.2 |
| Combination III | 24.00±3.3 | 47.54±13.9 |
Table 3: Mean and standard deviation of serum creatinine levels before and after treatment (n=5)
| Groups | Serum Creatinine (mg/dl) | |
| Day 0 | Day 19 | |
| Positive control | 0.60±0.08 | 2.75±0.6 |
| Negative control | 0.63±0.05 | 2.90±0.6 |
| Combination I | 0.46±0.09 | 3.20±0.6 |
| Combination II | 0.50±0.01 | 3.00±0.5 |
| Combination III | 0.58±0.08 | 3.30±0.3 |
Tables 2 and 3 illustrate that an increase in BUN and creatinine were significant. At day 19 compared to BUN and creatinine baseline on day 0, they had increased approximately 5 times. In the ANOVA test, it was found that there were no significant differences in BUN and creatinine levels on day 19 among groups (p > 0.05). This means that both glibenclamide and combination extract I, II, and III were not able to prevent kidney damage caused by alloxan administration.
Thin Layer Chromatography Profiles
On examination of the chemical constituents, test data includes the following results:
Ethanolic extracts of A. bilimbi L.
On examination TLC with silica gel GF254 plate and mobile phase toluene: ethyl acetate: methanol: glacial acetic acid (7.5 : 1.5 : 0.8 : 0.2) obtained the following results:
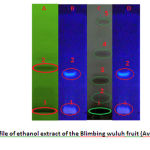 |
Figure 1: TLC profile of ethanol extract of the Blimbing wuluh fruit (Averrhoa bilimbi L.) |
(A) the appearance of under UV254dan (B) appearance under UV366 (C) derivatization with vanilin H2SO4 (D) derivatization with sitroborat
From Figure 1, several compounds may be identified and are listed in Table 4.
Table 4: Separation of ethanolic extract of Averrhoa bilimbi L fruit
| Detection | No | hRf | Description of colour | Chemical compound |
| UV 254 | 1 | 0 | Strong quenching | |
| 2 | 50 | Strong quenching | ||
| UV366 | 1 | 0 | Yellow fluorescence | Flavonoid |
| 2 | 47.5 | Yellow fluorescence | Flavonoid | |
| Vanilin H2SO4 | 1 | 0 | Purple black | Terpenoid |
| 2 | 20 | Purple black | Terpenoid | |
| 3 | 45 | Purple black | Terpenoid | |
| 4 | 70 | Purple black | Terpenoid | |
| 5 | 95 | Purple black | ||
| Sitroborat | 0 | Yellow fluorescence | Flavonoid | |
| 47.5 | Yellow fluorescence | Flavonoid
|
Ethanolic extract of C. roseus G
TLC examination results with silica gel GF254 plate and chloroform mobile phase: methanol (9.5 : 0.5), (I) appearance under UV254 and (II) under UV366 appearances (III) derivatization with vanilin H2SO4 (IV) derivatization with sitroborat obtained the results shown in Figure 2.
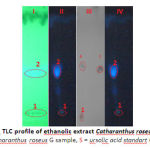 |
Figure 2: TLC profile of ethanolic extract Catharanthus roseus G leaves |
A= Catharanthus roseus G sample, S = ursolic acid standart (hRf= 55)
From Figure 2, several compounds may be identified and are listed in Table 5.
Table 5: Separation of ethanolic extract C. roseus G
| Detection | No | hRf | Description of colour | Chemical compound |
| UV 254 | 1 | 0 | Weak quenching | |
| 2 | 50 | Blue | ||
| UV366 | 1 | 0 | Blue fluorescence | Flavonoid |
| 2 | 50 | Blue fluorescence | Flavonoid | |
| Vanilin H2SO4 | 1 | 0 | Purple black | Terpenoid |
| 2 | 55 | Purple | Ursolic acid | |
| Sitroborat | 1 | 0 | Yellow fluorescence | Flavonoid |
| 2 | 50 | Yellow fluorescence | Flavonoid |
Discussion
Indonesia is unusually rich in medicinal plants. Among these medicinal plants, A. bilimbi L. fruit and C. roseus G. are often used for traditional medicinal purposes. In this study, the combination A. bilimbi L and C. roseus G dose IV (40:80 mg/200 g BW) and group V (80:40 mg/200 g BW) had the effect of lowering blood glucose, but were unable to prevent increasing BUN and creatinine levels in male rats induced by alloxan.
In previous studies, ethanol extract of Blimbing wuluh have had a hypoglycemic effect, anti-lipid peroxidative, antiatherogenic, and antihypertriglyceridemia in rats induced by streptozotosin. 32,33 The data indicates that ethanol extract dosage of 125 mg/kgBW was able to decrease blood glucose and triglyceride levels by 30-50%, and increases HDL by 60% compared to aquadest control.
The effect of lowering blood glucose levels in this study is consistent with the previous study. Treatment with the water fraction of ethanol extract dosage of 125 mg/kgBW significantly decreased blood glucose and trigliserida in rats induced by streptozotosin with a high fat diet. 34 Treatment by hydroalcoholic extract of leaves of Averrhoa carambola L. could lower blood glucose level on male Wistar rats by p 0.05.35
The addition of C. roseus G. can decrease blood glucose levels more than dosages without tapak dara. The combination A. bilimbi L. fruit 40 mg/200 gBW and C. roseus G. leaves 80 mg/200 gBW proved more effective in lowering blood glucose than the others. This research is consistent with research Som Nath Singh et al. performed, which stated that the dichloromethane extract of ethanol (1:1) 500 mg/kgBW dose is capable of lowering blood glucose in rats induced streptozotosin for 7 and 15 days with a 48.6% and 57.6% reduction.36
The study was also consistent with studies from Benjamin et al. (2004), stating that the water extract of dried leaves Tapak Dara was able to lower blood glucose levels by 60% .37 The extract of dichloromethane and methanol (1:1) extracts (500 mg/body weight) for 20 days of leaf Catharanthus roseus decreased blood glucose levels on alloxan induced diabetic rats.38
In this study, ethanolic extract of A.bilimbi L. fruit contained flavonoids and terpenoids. This is consistent with other researchers who stated that A.bilimbi L. fruit extract contains flavonoids, saponins and terpenoids.38,39 The chemical content of A.bilimbi L. reported amino acids, citric acid, cyanidin-3-O-β-D-glucoside, phenolics, potassium, and vitamin A. 40 The constituents of C. roseus G. leaves from this research are flavonoid, terpenoid, and ursolic acid. The study by Fereres at al. reported that besides alkaloids, other compounds of C. roseus are flavonoids and anthocyanins. Fifteen flavonol glycosides were identified from seeds, stems, leaves and flowers of C. roseus.41The study by Inte et al. (1998) notes that the chloroform extract of dried leaves of catharantus roseus contains ursolic acid, vindoline, oleanolic acid, and squalene.42
Conclusion
The combination of ethanolic extract of belimbing wuluh (A. bilimbi L.) fruit and Tapak dara (C. roseus G) leaves with dosages of 40 : 80 mg/200 gBW and 80 : 40 mg/200 gBW are effective for lowering blood glucose levels on day 7. On days 9, 13, and 19, combining dosages of 40:40 mg/200 gBW; 40:80 mg/200 gBW; and 80:40 mg/200 gBW are effective in lowering blood glucose (p < 0.05). All rats, BUN and creatinine levels increased on day 19. There is no significance difference in BUN and creatinine levels among treatment groups. Combinations of extract were not able to prevent kidney damage which was induced by alloxan (p > 0.05).
The chemical composition of 70% ethanolic extract Blimbing wuluh (A. bilimbi L.) are flavonoids and terpenoids. The chemical composition of 70% ethanolic extract of leaves of Tapak dara (C. roseus G) are ursolic acid, flavonoids, and terpenoids.
Acknowledgements
The authors would like to thank to the Department of Higher education of Indonesia as our funders.
References
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes care. 34, supplement 1:s62-s70 (2011).
- Wild S, Roglic G, Green A, Sicree R, King H.Global Prevalence of diabetes. Estimates for the year 2000 and projections for 2030. Diabetes care. 27 (5):1047-1054 (2004).
- Soeryoko, H, 25 Tanaman obat ampuh penakluk diabetes mellitus, Jakarta Andi Publisher. pp 1-125 (2011).
- Kumar KA, Gousia SK, Anupama M. And Latha JNL. A Review on Phytochemical Constituents and Biological Asays of Averhoa bilimbi. Int J Pharm Pharmac Sci 3(4): 136-139 (2013).
- Sutrisna EM, & Ermawati S, Mulyadin, Putro MAS. Uji Praklinis Efek Hipoglikemik Blimbing Wuluh (Averrhoa bilimbi ) Dan Daun Tapak Dara (Catharanthus roseus G). Pharmacon. 13(1): 37-40 (2012).
- Nagmoti DM, Yeshwante SB, Wankhede SS, Juvekar AR. Hepatoprotective effect of Averrhoa bilimbi linn. against carbon tetrachloride induced hepatic damage in rats. 3: 1-6 (2010).
- Ambilli S, Subramoniam A, Nagarajan NS. Studies on the Antihyperlipidemic Properties of Averrhoa bilimbi Fruit in Rats. Planta 75(1) 55-58 (2009).
- Nasim SA, Ilah A, Ahmad IZ, Khan SA, Mujib A, and Sharma MP. Catharanthus roseus (L.) Don. An Important Drug : It’s Applications and Production. IJCP. 4 (12): 1-16 (2010).
- Ali R, Hossain M, Runa JF, Hasanuzzaman. Preliminary cytotoxic activity of different extracts of Averrhoa bilimbi (fruits). Int Current Pharmaceutical J. 2(3): 83-84 (2013).
- Brandao M, Botelho M, Krettli E. Antimalerial Experimental Chemotherapy using Natural Products. Cienc Cult. 37(7): 1152-1163 (1985).
- De Mello JF: Plants in traditional medicine in Brazil. J Ethnopharmacol. 2(1): 49-55 (1980).
- Holdsworth DK: Traditional medicinal plants of Rarotonga, Cooks Islands Part I. Int J Crude Drug Res. 28(3): 209-218 (1990).
- Swanston-Flatt SK, Day C, Flatt PR, Gould BJ, Bailey CJ. Glycaemia effects of traditional European plant treatments for diabetes studies in normal and streptozotocin diabetic mice. Diabetes Res. 10: 69-73 (1989).
- Rahman AU. Some approaches to the study of indigenous medicinal plants. Bull Islamic Med. 2: 562-568 (1982).
- Cordell GA, Weiss SG, Farnsworth NR. Structure elucidation and chemistry of Catharanthus alkaloids. XXX. Isolation and structure of vincarodine. J Org Chem. 39 (4): 431-434 (1974).
- Johnson IS, Wright HF, Svoboda GH, Vlantis J. Antitumor principles derived from Vinca rosea Vincaleukblastine and leurosine. Cancer Res. 20: 1016 (1960).
- El-Sayed A, Cordell GA. Catharanthus alkaloids XXXIV. Catharanthamine, a new antitumor bisindole alkaloid from Catharanthus roseus. J Nat Prod. 44: 289-293 (1981).
- Murugavel TA, Ruknudin ST, Akbarsha MA. Antifertility effect of Vinca rosea (Linn.) leaf extract on male albino mice – a sperm parametric study. Curr Sci. 58(19): 1102- 1103 (1989).
- Chopra IC, Jamwal KS, Chopra CL, Nair CP & Pillay PP. Preliminary pharmacological investigations of total alkaloids of Lochnera rosea (Rattonjot). Indian J Med Res. 47: 40-43 (1959).
- Rai MK, Upadhyay S. Screening of medicinal plants of Chhindwara district against Trichophyton mentagrophytes: A casual organism of Tineapedis. Hindustan Antibiot Bull. 30 (1/2): 33-36 (1988).
- Chile SK, Saraf M, Barde AK. Efficacy of Vinca rosea extract against human pathogenic strains of Trichophyton rubrum Indian Drugs Pharm Ind. 16(1): 31-33 (1981).
- Kulkarni R, Ravindra Resistance to Pythium aphanidermatum in diploid and induced autotetraploids of Catharanthus roseus. Planta Med. 54 (4): 356-359 (1988).
- Jarald EE, Joshi SB, Jain DC, Edwin S Biochemical evaluation of the hypoglycemic effects of extract and fraction of Cassia fistula in alloxan-induced diabetic rats. Indian J Pharm Sci. 74(5):427-434 (2013).
- Viswanathaswamy AHM, Koti BC, Gore A, Thippeswamy AHM, Kulkarni RV. Antihyperglycemic and antihyperlipidemic activity of Plectranthus amboinicuson normal and alloxan-induced diabetic rats. Indian J Pharm Sci. 73 (2): 135-149 (2011).
- Kumar S,Malhotra R, Kumar Antidiabetic and free radicals scavenging potential of Euphorbia hirta flower extract. Indian J Pharm Sci. 72(4): 533-537 (2010).
- Dhanabal SP,Marugaraja MKM, Suresh, B. Antidiabetic activity of Clerodendron phlomoidis leaf extract in alloxan-induced diabetic rats. Indian J Pharm Sci. 70(6): 841-844 (2008).
- Belhekar SN, Chaudhari PD, Saryawanshi JS, Mali KK, Pandhare, RB. Antidiabetic and antihyperlipidemic effects of thespesia populnea fruit pulp extracts on alloxan-induced diabetic rats. Indian J Pharm Sci. 75(2):217-221 (2013).
- Mohammed A, Adelaiye AB, Abubakar, MS and Abdurahman EM. Effects of aqueous extract of Ganoderma lucidum on blood glucose levels of normoglycemic and alloxan-induced diabetic wistar rats. J Med Plants Res.1(2): 034-037 (2007).
- Bathini P, Kameshwari L,& Vijaya N. Antidiabetic effect of 2 nitro enzimidazole in alloxan induced diabetic rats. IJBCP. 2(6): 814-817 (2013).
- Kulandaivel S, Bajpai P and Sivakumar T. Anti-hyperglycemic activity of Trichosanthes tricuspidata root extract. Bangladesh J Pharmacol.8: 305-310 (2013).
- Yakubu MT and Ogunro OB.Effects of aqueous extract of Fadogia agrestis stem in alloxan-induced diabetic rats. Bangladesh J Pharmacol. 9: 356-363 (2014).
- Pushparaj P, Tan CH, Tan BK. Effects of Averrhoa bilimbi leaf extract on blood glucose and lipids in streptozotocin-diabetic rats. J Ethnopharmacol. 72(1-2):69-76 (2000).
- Puspharaj PN, Evaluation Of The Anti-Diabetic Properties Of Averrhoa Bilimbi In Animals With Experimental Diabetes Mellitus, Thesis. pp 18-21. National University Of Singapore.Singapora(2004).
- Tan BKH, Tan CH, Pushparaj PN. Anti-diabetic activity of the semi purified fractions of Averrhoa bilimbi in high fat diet fed-streptozotocin-induced diabetic rats. Life Sci. 76(24):2827-2839 (2005).
- Ferreira EB, Fernandes LC, Galende SB. Diogenes A. G. CortezI; Roberto B. Bazotte, Hypoglycemic effect of the hydroalcoholic extract of leaves ofAverrhoa carambola (Oxalidaceae). Rev Bras Farmacogn. 18(3):339-343 (2008).
- Singh SN, Vats P, Suri S, Shyam R, Kumria MML, Ranganathan S, Sridharan, K. Effect of an antidiabetic extract of Catharanthus roseus on enzymic activities in streptozotocin induced diabetic rats. J Ethnopharmacol. 76: 269–277 (2001).
- Benjamin BD, Kelkar SM, Pote MS, Kaklij GS, Sipahimalani AT & Heble MR, Catharanthus roseus cell culture: Growth, alkaloid synthesis and antidiabetic activity. Phytother Res. 8(3): 185-186 (1994).
- Jayanthi M, Sowbala1 N, Rajalakshmi G,Kanagavalli U, Sivakumar V, Study Of Anti Hyperglycemic Effect Of Catharanthus Roseus In Alloxan Induced Diabetic Rats. Int J Pharm Pharmac Sci. 4: 19-25 (2009).
- Wahab NHBA, Wahid MEBA, Taib MBt , Wan Mohd Zain WZBt and Anwar SABt. Phytochemical screening and antimicrobial efficacy of extracts from Averrhoa bilimbi (Oxalidaceace) fruits against human pathogenic bacteria. J of Pharmacognosy. 1(1): 64-66 (2009).
- Goh SH, Chuah CH, Mok JSL, Soepadmo E. Malaysian medicinal plants for the treatment of cardiovascular diseases. Pelanduk Publication, Kuala Lumpur. pp.63. 1995.
- Ferreres F, Pereira DM, Valentao P, Andrade PB, Seabra RM, Sottomayor M. New phenolic compounds and antioxidant potential of Catharanthus roseus. J Agric Food Chem. 56:9967−74 (2008).
- Inte, VML, Ragasa CY, Rideout, JA. Triterpenes, hydrocarbons and antimutagenic alkaloid from Catharanthus roseus (Apocynaceae). Asia life sci. 7(1): 11-21 (1998).







