Manuscript accepted on :
Published online on: 25-11-2015
Plagiarism Check: Yes
Shadi Akhavan1, Mahnaz Qomi*, Gayaneh Charmahali2, Sharareh Akhavan2
1Department of Medicinal Chemistry (Pharmaceutical Sciences Research Center), Pharmaceutical Sciences Branch, Islamic Azad University, Tehran, Iran. 2Department of applied chemistry Islamic Azad University , Shahr-e-rey Branch, shahr-e-rey, Iran
DOI : https://dx.doi.org/10.13005/bpj/640
Abstract
Zopiclone is used to treat sleeping problems (insomnia). In the current work, for the first time, a three-phase hollow-fiber liquid-phase microextraction combined with HPLC-UV was proposed for the determination of zopiclone in urine samples. Different factors that can affect the extraction process such as extraction solvent, acceptor and donor phase composition, salt addition, stirring rate, extraction time and extraction temperature were optimized. Under the optimum conditions, detection and quantitation limits obtained 53.9 and 161.7ng mL-1, respectively, and enrichment factors 116 were obtained. The calibration curves were linear within the range of 161.7-2000 ng mL-1 with estimation of coefficient higher than 0.9990. Within a day and between day RSDs were 5.51% and 5.34%, respectively. Finally, the proposed method was applied to the detection and determination of zopiclone in human urine sample.
Keywords
Zopiclone; High performance liquid chromatography; Hollow fiber based liquid phase microextraction; Microextraction
Download this article as:| Copy the following to cite this article: Akhavan S, Qomi M, Charmahali G, Akhavan S. Hollow IFber Based Liquid Phase MicroextractionCombined High Performance Liquid Chromatograph for the Determination Trace Amounts of Opiclone in Biological Fluids. Biomed Pharmacol J 2015;8(1) |
| Copy the following to cite this URL: Akhavan S, Qomi M, Charmahali G, Akhavan S. Hollow IFber Based Liquid Phase MicroextractionCombined High Performance Liquid Chromatograph for the Determination Trace Amounts of Opiclone in Biological Fluids. Biomed Pharmacol J 2015;8(1). Available from: http://biomedpharmajournal.org/?p=1584 |
Introduction
Zopiclone is used to treat sleeping problems (insomnia) [1-5]. The chemical structure and phisico-chemical properties of the drugaresummrized in Table 1[6].Zopiclone belongs to a class of medicines commonly called Z drugs. It works by acting in the way messages are sent to your brain, which help you to sleep. It reduces the time it takes for you to fall asleep and increases the length of time you spend sleeping[7]. Along with their useful effects, most medicines can cause unwanted side-effects, although not everyone experiences them. Daytime drowsiness, dizziness, lightheadedness, bitter taste, dry mouth, headache or stomach upset may occur the first few days as you took the medication[8]. Several methods have been presented for detection and determination of zopiclone until now. There are some methods for determination of zopicloneconcentration in biological fluids including liguid chromatography [9-15], Liquid chromatography–mass spectrometry[16], HPLC using fluorescence detection [17], capillary electrophoresis[18-20], gas chromatography [21, 22], spectrophotometric techniques [23] and voltametrics methods [24]. In all of reported methods, for determination of the zopiclone in biological samples, sample preparation steps are necessary. Additionally, in some cases protein precipitation and derivatization steps should be used [19]. Therefore, it is necessary to introduce a new, fast, simple and sensitive method that improves detection and determination of zopiclone in biological fluid samples.
Tothe best of our knowledge,microextraction technique has not been introduced for extraction and preconcentration of zopiclone from biological fluids. for the first time, in this work, three phasehollow fiber based liquid phase microextraction (HF-LPME) followed by HPLC with ultraviolet (UV) detection was optimized and validated for detection and determination of zopiclone in biological samples.
For the first time, LPME was reported by Dasgupta[25] and Cantwell [26] in 1996, which is a novel miniaturized sample preparation technique taken from traditionalliqid-liquid extraction. Hollow fiber liquid phase microextraction (HF-LPME) as one of LPME methods was introduced in 1999 [27, 28]. HF-LPME is easy, cheap and environmentally friendly which allows multiple sample processing steps, for example, extraction, clean up, and analyte enrichment to be performed in a single step [29]. HF-LPME divided into two-phase HF-LPME and three-phase HF-LPME. In three-phase hollow fiber extraction, analytes are extracted from an aqueous sample into an organic phase, and then back extracted into a separate aqueous phase.[30-37].
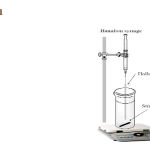 |
Figure 1: Schematic diagram of proposed HF-LPME setup. |
In the current work, the efficacy of different variables on HF-LPME efficiency were investigated and optimized. After optimization, the method followed by HPLC-UV was used for extraction and determination of zopiclone in urine sample.
Experimental
Chemicals, standard and stock solutions
Zopiclone standard wasobtained from Drug and Food Administration (Tehran, Iran).All of chemicals used were of analytical-reagent grades or better. 1-Octanol, decanol, isobutylmethyl ketone, n-heptan all from Merck (Darmstadt, Germany), were used as organic membrane solvents.HPLC grade acetonitrile and methanolwere purchased from merck.Sodium hydroxide and sodium chloride were obtained from Sigma–Aldrich (St. Louis, MO, USA).
A PPQ3/2 Accurel polypropylene hollow fiber from Membrana (Wuppertal, Germany) with a pore size of 0.2 µm, the inner diameter of 0.6 mm and wall thickness of 200 µm was used for the extraction process.Ultrapure water was obtained from a Milli-Q water purification system from Millipore (Madrid, Spain).
A 1000 mg L−1 stock solution of zopiclone was prepared in methanol and standard working solutions were prepared by spiking the proper amount of the stock solution in pure water.
Instruments and equipment
AYounglin YL9100 HPLC (Seoul, Korea) containing a Quaternary9110 HPLC pump (Seoul, Korea), a 4-channel mixing valve with a 20 µL sample loop, YL9101 vacuum degasser and a YL 9120 UV-Vis detectorwas usedfor separation and detection of the target analyte. Chromatography data were recorded and analyzed using Younglin Auto Chro 3000 software.The separations were performed on an ODS-3 column (150 mm × 4.6 mm, with particle size of 5 µm) from MZ-Analysentechnik (Mainz, Germany).The mobile phase consisted of a mixture of methanol and acetonitrile (35:65), under isocratic condition. The flow rate of the mobile phase was set at 1.2 mL min-1. Total analysis time was 8 min.The injection volume was 20 µL for all of the samples and detection was performed at a wavelength of 304 nm.
A MR Hei-standard magnetic stirrer from Heidolph (Schwabach, Germany) was used for agitation of sample solution. GPHR 1400 digital pH meter from Greisinger (Regenstauf, Germany) was used for pH measurements.
Extraction procedure
A fresh 8.0 cm length of hollow fiber was cut and washed with acetone in an ultrasonic bath for 10 min and dried at room temperature. Fifteen milliliter of sample solution was filled into a 20 mL vial which was placed on a magnetic stirrer plate for agitation of sample solution during the extractions. Extraction process was shown in Fig. 1. The hollow fiber was placed at the end of 100 µL Hamilton syringe needle that filled with acceptor phase, and subsequently dipped for a 10 s period into the organic solvent (1-Octanol) used for impregnation. After impregnation, excess amount of organic solvent washed with distilled water for 30 s, and 20 µL of acceptor solution with pH=3.0 was injected into the hollow fiber. First, 80 μL of acceptor solution was flushed out of the fiber in order to remove any organic solvent remaining inside the lumen of hollow fiber, and then 20 μL acceptor solution was remained in the lumen of hollow fiber, and the lower end of the hollow fiber was mechanically sealed by means of a piece of aluminum foil. Subsequently, the fiber was placed in the sample solution. During extraction, the solution was stirred at 1000 rpm. After extraction, the acceptor solution was collected into a micro-vial by Hamilton syringe and finally, 20 µL of acceptor solution was injected into the HPLC instrument for analysis.
Real sample analysis
A urine sample was collected from healthy young volunteer. The sample was stored at −4˚Cin the dark until analysis without further sample pretreatment and thawed and shaken before extraction
Calculation of preconcentration factor
The preconcentration factor (PF) was defined as the ratio of the final analyte concentration in the acceptor phase (Cf,a) and the initial concentration of analyte (Ci,s) in the sample solution:

where Cf,a was calculated from a calibration graph obtained by direct injection of analytes standard solutions.
Results and Discussion
Selection of the extraction solvent
The selection of a suitable extraction solvent is a important step in HF-LPME for achieving high extraction efficiency. The composition of chosen solvent should be compatible with the used fiber, immiscible with acceptor and donor phases, have low volatilityin order to prevent loss of solvent during the extraction, and should also have good affinity for the analytes[38]. In the current work, four different organic solvent,1-Octanol, n-decanol, isobutyl methyl ketoneand n-heptanewere tested as extraction solvent. The corresponding results obtained for the extraction solvents studied are shown in Fig. 2A. As shown in Fig. 2A, the target analyte exhibited the highest peak area when 1-octanol was used. Therefore, 1-octanol was chosen as the extraction solvent for subsequent analysis.
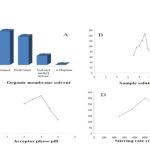 |
Figure 2: Optimization of (A) organic membrane solvent, (B) sample solution pH, (C) acceptor phase pH and (D) stirring rate for extraction of zopiclone. |
The pH in sample solution and acceptor phase
Zopiclone is a weak basic drug with pKa value about 6.89. In order to obtain high extraction efficiency of the target analytes by the HP-LPME, they should be first transformed to their neutral molecular forms, which could be done by the addition of sodium hydroxide to the samples. This step dramatically facilitates the diffusion of the zopiclone through the liquid membrane and then permeation to the acidic acceptor solution, where they will be irreversibly trapped because of the formation of the resulting ionic species. In order to investigate the effect of the donor phase pH, donor pH was tested in the basic pH from 7.0 to 12.0. As shown in Fig. 2B, the highest extraction efficiency for zopiclone was obtained using pH=9.0. Therefore, pH=9.0 was used for subsequent experiments.
To extract basic compounds, the acceptor phase should be acidic to provide high solubility for basic analyte and to ionize them to prevent reentry into the organic phase. For this purpose, pH of acceptor phase was studied from two to four. Results in Fig. 2C show that highest extraction efficiency was obtained at pH=3.0. Therefore, pH=3.0 was used for subsequent experiments.
Effect of stirring rate
The stirring rate is another significant parameter that can greatly influence the extraction efficiency. Agitation of the sample makes the mass transfer equilibrium between the phases faster and hence reduces extraction time. Stirring facilitates analyte diffusion from donor phase into the acceptor phase. In this work, the effect of the stirring rate onthe extraction efficiency was tested in the range of 500 to 1500 rpm. It can be seen that when the stirring ratewas increased from 500 to 1000 rpm, the extraction efficiency oftarget analytewas enhanced. However, another increasing in stirring rate over 1000 rpm decrease extraction efficiecye due to the loss of organic phase and air bubble formation around the hollow fiber under the higher stirring rate.. Hence,according to Fig. 2D, astirring rate of 1000 rpm was selected as the optimum stirring rate for nextexperiments.
Effect of salting-out
Commonly, the addition of a small amount of salt to the sample solution produces a salting-out effect that decrease the solubility of analytes in the aqueoussolution. However, by the addition of salt, the aqueous solution viscosity would increase, which lead to difficult mass transfer and decrease extraction efficiency. In current work, the effect of different concentrations m/v) of NaCl in the range of 0% to 10% to extract target analytewas tested. As shown inFig. 3A, Theextraction efficiency increased when the NaCl concentration was increased from 0 to 4% (w/v). Over4% salt addition, decreased extraction efficiency. Thus, 4% (w/v) addition of salt was selected for the subsequent experiments.
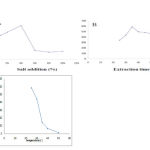 |
Figure 3: Optimization of (A) salt addition effect, (B) extraction time and (C) temperature for extraction of zopiclone. |
Effect of extraction time
The extraction time is an important parameter in HF-LPME procedure. HF-LPME is a non-exhaustive extraction method as the analytes are partitioned between the donor and the acceptor phases until the equilibrium is established. In current work, the efficiency of extraction time in zopiclone extraction was tested in the range of 20 to 60 min.as shown in Fig. 3B,the extraction efficiency increased dramatically with the increasing of the extraction time up to 35 min. Another increasing in the extraction time to 60 min,lead to rapidly decrease in the extraction recovery due to organic membrane dissolution in the sample solution. Therefore, 35 min was selected as the best extraction time in the subsequent experiments.
Effect of temperature
The effect of temperature on the extraction efficiency of zopiclone by HF-LPME was investigated over a temperature range of room temperature to 50 °C.Increasing of temperature from 4 to 25 °C increase extraction efficiency, butwhen the extraction temperature was higher than 25 °C, decreased the extraction efficiency seriously due to air bubble formation around the hollow fiber. Therefore, the temperature of sample temperature of 25 ºC was selected as the optimum temperature in current work.As shown in Fig. 3B,
As a consequence, the optimal conditions were attained by using pH=3.0 and pH=9.0 as the acceptor and donor phases composition, respectively and using 1000 rpm for agitation for 35 min. Additionally, the organic membrane composition was 1-Octanol. Fifteen milliliter of sample solution, 4% addition of salt and sample temperature of 20 °C was selected as the optimal condition for zopiclone extraction.
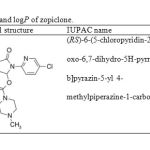 |
Table 1: Chemical structures, pKa and logP of zopiclone. |
Validation of the proposed method
To evaluate the practical applicability of the proposed HF-LPME method, figures of merit were studied using standard solutions of the analyte in a drug-free urine samples. Optimal condition was applied to find out linearity, repeatability, and LODs of this method. The figures of merit for the optimized method are summarized in Table 2.The calibration curvewas linear in the range of 161.7–0000 µg L-1 with coefficient of determination (r2)more than 0.9990. The relative standard deviations (RSD %) for extraction and determination of zopiclone were less than 5.51% and 5.34% for within day and between day experiment, respectively. LODs less than 53.9 µg L-1 wasviewed for target analyte. PF values higher than 116-fold were obtained for the extraction of zopiclone at the concentration level of 1.0mg L-1. The LOD and LOQ were calculated as 3× S/N and 10× S/N, respectively.
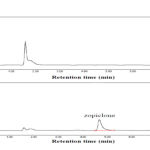 |
Figure 4: Chromatograms obtained after HF-LPME extraction of urinesample ((A) non-spiked sample and (B) spiked sample at a concentration level of 1.0 mg L-1). |
Table 2: Figures of merit of HF-LPME in drug distilled water sample.
| LOD (µgmL-1) | LOQ (µg mL-1) | Linearity
(µgmL-1) |
R2 | PFa
|
RSD% b R% | |
| Within day | Between day | |||||
| 5.39*10-6 | 16.17*10-6 | 0.01-2 | 0.9990 | 116 | 5.51 | 5.34 15 |
| a Drugs were present at 1.0µg mL-1.
b Within day and between day RSDs% were obtained by four replications. |
||||||
Analysis of real sample
HF-LPME is a powerful method for detection and determination of zopiclone from untreated biological fluids. Therefore, the optimal conditions of HF-LPME were used for extraction of zopiclone from human urine sample. To reduce matrix effects calibration curve wasplotted in drug free urine sample.
Table 3: Determination of zopiclone in urine sample.
| Sample | Creal
(µg mL-1) |
Cadded
(µg mL-1) |
Cfound
(µg mL-1) |
RSD%
(n = 4) |
|
| Urine | nda | 1 | 0.4 | 3.4 | |
| a Not detected | |||||
Drug-free human urine sample was spiked with the target drug and extraction was accomplished after dilution of urine samples (1:3) and the addition of proper amount of NaOH solution to achieve pH 9.0. The results are summarized in Table3. RSD% values less than 3.4% confirm the acceptable precision of proposed HF-LPME method. Fig. 4 shows typical chromatograms of blank and spiked urine sample after applying the proposed HF-LPME method.
In comparison to other alternative methods recently reported for the determination of zopiclone in biological samples, the proposed method has in general, lower LOD, higher PF, good precision, suitable linear dynamic range and sensitivity. Comparison results of the proposed method with different existing methods is provided in Table 4.
Table 4: Comparison of the HF-LPME with other analytical techniques for determination of zopiclone.
| Analytical method | Sample preparation method | Sample | LOD
(µg mL-1) |
Linearity
(µg mL-1) |
RSD% | Ref. |
| HPLC | HF-LPME | Urine | 5.39*10-6 | 10-2000 | 5.34 | This work |
|
GC-MS |
LLE | Plasma | 0.4 | 0.002 -1.3 | 4.6 | [39] |
|
CE
|
LLE | Urine | R(-)0.509
R(+)0.501 |
0.57-30 | R(-)5.5
R(+)6.6 |
[40] |
| HPLC-UV
|
LLE | Tablet | 0.02 | 1–6 | 6.7 | [40] |
| ADSV | – | Urine | 2.78*10-7 | 6*10-7-2*10-5 | 1.0 | [41] |
|
HPLC-ESI- MS-MS |
SPE | Plasma | 0.98*10-3 | 0.5*10-3-0.15 | 9.7 | [42] |
|
HPTLC |
– | Serum | 52.8*10-2 | 0.1-0.4 | 1.49 | [43] |
Conclusions
In the current work, for the first time, we have developed a three-phase HF-LPME method combined with HPLC–UV for the sample preparation and determination of zopiclone in urine sample. The optimized three phase HF-LPME method provides enrichment of the analyte and cleanup processes in a single step, and the final extract being compatible with direct injection into the HPLC instrument. Acceptable LODs and RSDs, high preconcentration factor and extraction efficiency, and good linearity ranges were obtained with the proposed method. The developed method is a simple alternative that can be successfully applied for detection and determination of zopiclone in biological samples.
References
- T.R.M. Leufkens, J.G. Ramaekers, A.W. De Weerd, W.J. Riedel, A. Vermeeren, Residual effects of zopiclone 7.5 mg on highway driving performance in insomnia patients and healthy controls: A placebo controlled crossover study, Psychopharmacology, 231 (2014) 2785-2798.
- S.X. Wang, L. Qin, Y. Tang, H.D. Wu, S.M. Lin, G.L. Xu, Meta-analysis on effectiveness and safety of zaleplon compared with zopiclone in treatment of insomnia, Journal of Jilin University Medicine Edition, 39 (2013) 104-108.
- G.T. Lesser, Treatment of chronic insomnia with cognitive behavioral therapy vs zopiclone [2], Journal of the American Medical Association, 296 (2006) 2435-2436.
- M.G. Terzano, M. Rossi, V. Palomba, A. Smerieri, L. Parrino, New drugs for insomnia: Comparative tolerability of zopiclone, zolpidem and zaleplon, Drug Safety, 26 (2003) 261-282.
- S. Tsutsui, A double-blind comparative study of zolpidem versus zopiclone in the treatment of chronic primary insomnia, Journal of International Medical Research, 29 (2001) 163-177.
- F.a.D. Administration, Guidance for Industry; Bioanalytical Method Validation, , Rockville, U.S. Department of Health and Human Services, 2001.
- A. Döble, T. Canton, C. Malgouris, J.M. Stutzmann, O. Piot, M.C. Bardone, C. Pauchet, J.C. Blanchard, The mechanism of action of zopiclone, European Psychiatry, 10, Supplement 3 (1995) 117s-128s.
- C.Y. Liu, Y.Y. Yang, E.K. Yeh, Efficacy and side effects of zopiclone and triazolamin in the treatment of Chinese patients with insomnia – A double blind cross-over study, International Medical Journal, 4 (1997) 45-48.
- B. Paw, G. Misztal, Determination of zopiclone in tablets by HPLC and UV-spectrophotometry, Journal of Pharmaceutical and Biomedical Analysis, 23 (2000) 819-823.
- J.P. Bounine, B. Tardif, P. Beltran, D.J. Mazzo, High-performance liquid chromatographic stability-indicating determination of zopiclone in tablets, Journal of Chromatography A, 677 (1994) 87-93.
- C. Fernandez, F. Gimenez, B. Baune, V. Maradeix, A. Thuillier, R. Farinotti, Determination of the enantiomers of zopiclone and its two chiral metabolites in urine using an automated coupled achiral—chiral chromatographic system, Journal of Chromatography B: Biomedical Sciences and Applications, 617 (1993) 271-278.
- M.J. Royer-Morrot, M. Rambourg, I. Jacob, P. Bauer, R.J. Royer, Determination of zopiclone in plasma using column liquid chromatography with ultraviolet detection, Journal of Chromatography B: Biomedical Sciences and Applications, 581 (1992) 297-299.
- C. Fernandez, B. Baune, F. Gimenez, A. Thuillier, R. Farinotti, Determination of zopiclone enantiomers in plasma by liquid chromatography using a chiral cellulose carbamate column, Journal of Chromatography B: Biomedical Sciences and Applications, 572 (1991) 195-202.
- A. Le Liboux, A. Frydman, J. Gaillot, Simultaneous determination of zopiclone and its two major metabolites (n-oxide and n-desmethyl) in human biological fluids by reversed-phase high-performance liquid chromatography, Journal of Chromatography B: Biomedical Sciences and Applications, 417 (1987) 151-158.
- L.G. Miller, B.W. Leduc, D.J. Greenblatt, Determination of zopiclone in plasma by liquid chromatography with application to steady-state monitoring, Journal of Chromatography B: Biomedical Sciences and Applications, 380 (1986) 211-215.
- E. Eliassen, L. Kristoffersen, Quantitative determination of zopiclone and zolpidem in whole blood by liquid–liquid extraction and UHPLC-MS/MS, Journal of Chromatography B, 971 (2014) 72-80.
- R.N. El-Shaheny, A. Alattas, J.J. Nasr, N. El-Enany, F. Belal, Simultaneous determination of zopiclone and its degradation product and main impurity (2-amino-5-chloropyridine) by micellar liquid chromatography with time-programmed fluorescence detection: Preliminary investigation for biological monitoring, Journal of Chromatography B, 907 (2012) 49-55.
- T.G. Halvorsen, S. Pedersen-Bjergaard, K.E. Rasmussen, Liquid-phase microextraction and capillary electrophoresis of citalopram, an antidepressant drug, Journal of Chromatography A, 909 (2001) 87-93.
- S. Andersen, T.G. Halvorsen, S. Pedersen-Bjergaard, K.E. Rasmussen, L. Tanum, H. Refsum, Stereospecific determination of citalopram and desmethylcitalopram by capillary electrophoresis and liquid-phase microextraction, Journal of Pharmaceutical and Biomedical Analysis, 33 (2003) 263-273.
- G. Hempel, G. Blaschke, Enantioselective determination of zopiclone and its metabolites in urine by capillary electrophoresis, Journal of Chromatography B: Biomedical Sciences and Applications, 675 (1996) 139-146.
- F. Stanke, N. Jourdil, J. Bessard, G. Bessard, Simultaneous determination of zolpidem and zopiclone in human plasma by gas chromatography-nitrogen-phosphorus detection, Journal of Chromatography B: Biomedical Sciences and Applications, 675 (1996) 43-51.
- Y. Gaillard, J.-P. Gay-Montchamp, M. Ollagnier, Gas chromatographic determination of zopiclone in plasma after solid-phase extraction, Journal of Chromatography B: Biomedical Sciences and Applications, 619 (1993) 310-314.
- M.M. Abdelrahman, I.A. Naguib, M.R. El Ghobashy, N.A. Ali, Quantitative determination of zopiclone and its impurity by four different spectrophotometric methods, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 137 (2015) 617-624.
- S. Yılmaz, Adsorptive stripping voltammetric determination of zopiclone in tablet dosage forms and human urine, Colloids and Surfaces B: Biointerfaces, 71 (2009) 79-83.
- H. Liu, P.K. Dasgupta, Analytical Chemistry in a Drop. Solvent Extraction in a Microdrop, Analytical Chemistry, 68 (1996) 1817-1821.
- M.A. Jeannot, F.F. Cantwell, Solvent Microextraction into a Single Drop, Analytical Chemistry, 68 (1996) 2236-2240.
- S. Pedersen-Bjergaard, K.E. Rasmussen, Liquid−Liquid−Liquid Microextraction for Sample Preparation of Biological Fluids Prior to Capillary Electrophoresis, Analytical Chemistry, 71 (1999) 2650-2656.
- B. Ebrahimpour, Y. Yamini, A. Esrafili, Emulsification liquid phase microextraction followed by on-line phase separation coupled to high performance liquid chromatography, Analytica Chimica Acta, 751 (2012) 79-85.
- A. Gure, F.J. Lara, N. Megersa, A.M. García-Campaña, M. del Olmo-Iruela, Hollow-fiber liquid-phase microextraction combined with capillary HPLC for the selective determination of six sulfonylurea herbicides in environmental waters, Journal of Separation Science, 36 (2013) 3395-3401.
- Y.-Y. Chao, Y.-M. Tu, Z.-X. Jian, H.-W. Wang, Y.-L. Huang, Direct determination of chlorophenols in water samples through ultrasound-assisted hollow fiber liquid–liquid–liquid microextraction on-line coupled with high-performance liquid chromatography, Journal of Chromatography A, 1271 (2013) 41-49.
- T.S. Ho, J.L. Egge Reubsaet, H.S. Anthonsen, S. Pedersen-Bjergaard, K.E. Rasmussen, Liquid-phase microextraction based on carrier mediated transport combined with liquid chromatography–mass spectrometry: New concept for the determination of polar drugs in a single drop of human plasma, Journal of Chromatography A, 1072 (2005) 29-36.
- L. Hou, G. Shen, H.K. Lee, Automated hollow fiber-protected dynamic liquid-phase microextraction of pesticides for gas chromatography–mass spectrometric analysis, Journal of Chromatography A, 985 (2003) 107-116.
- A. Rodríguez, S. Pedersen-Bjergaard, K.E. Rasmussen, C. Nerín, Selective three-phase liquid phase microextraction of acidic compounds from foodstuff simulants, Journal of Chromatography A, 1198–1199 (2008) 38-44.
- Y. Wu, B. Hu, Simultaneous determination of several phytohormones in natural coconut juice by hollow fiber-based liquid–liquid–liquid microextraction-high performance liquid chromatography, Journal of Chromatography A, 1216 (2009) 7657-7663.
- L. Xia, B. Hu, Y. Wu, Hollow fiber-based liquid–liquid–liquid microextraction combined with high-performance liquid chromatography for the speciation of organomercury, Journal of Chromatography A, 1173 (2007) 44-51.
- J. Xiong, B. Hu, Comparison of hollow fiber liquid phase microextraction and dispersive liquid–liquid microextraction for the determination of organosulfur pesticides in environmental and beverage samples by gas chromatography with flame photometric detection, Journal of Chromatography A, 1193 (2008) 7-18.
- J. Zhang, T. Su, H.K. Lee, Development and application of microporous hollow fiber protected liquid-phase microextraction via gaseous diffusion to the determination of phenols in water, Journal of Chromatography A, 1121 (2006) 10-15.
- K.E. Rasmussen, S. Pedersen-Bjergaard, Developments in hollow fibre-based, liquid-phase microextraction, TrAC Trends in Analytical Chemistry, 23 (2004) 1-10.
- M. Baghdadi, F. Shemirani, Cold-induced aggregation microextraction: A novel sample preparation technique based on ionic liquids, Analytica Chimica Acta, 613 (2008) 56-63.
- L. Meng, X. Liu, B. Wang, G. Shen, Z. Wang, M. Guo, Simultaneous derivatization and extraction of free cyanide in biological samples with home-made hollow fiber-protected headspace liquid-phase microextraction followed by capillary electrophoresis with UV detection, Journal of Chromatography B, 877 (2009) 3645-3651.
- M. Rezaee, Y. Assadi, M.-R. Milani Hosseini, E. Aghaee, F. Ahmadi, S. Berijani, Determination of organic compounds in water using dispersive liquid–liquid microextraction, Journal of Chromatography A, 1116 (2006) 1-9.
- M. Ramos Payán, M.Á. Bello López, R. Fernández-Torres, J.A.O. González, M. Callejón Mochón, Hollow fiber-based liquid phase microextraction (HF-LPME) as a new approach for the HPLC determination of fluoroquinolones in biological and environmental matrices, Journal of Pharmaceutical and Biomedical Analysis, 55 (2011) 332-341.
- M.R. Payán, M.Á.B. López, R. Fernández-Torres, J.L.P. Bernal, M.C. Mochón, HPLC determination of ibuprofen, diclofenac and salicylic acid using hollow fiber-based liquid phase microextraction (HF-LPME), Analytica Chimica Acta, 653 (2009) 184-190.







