Manuscript accepted on :
Published online on: 22-11-2015
Plagiarism Check: Yes
Neda Jamshidi Shahmirzadi1*and Seyed Hosein Inanloo2
1Postgraduate student of Pediatric Dentistry, Islamic Azad University, Dental Branch, Tehran, IRAN 2Assistant Professor, Department of Operative Dentistry, Shahed Dental School, Tehran, Iran
DOI : https://dx.doi.org/10.13005/bpj/610
Abstract
The novel bisphenol-A glycidyl methacrylate (Bis-GMA)/triethylene glycol dimethacrylate (TEGDMA) /hydroxyapatite (HAp) nanofibrous dental resins were successfully fabricated and the influence of HApnanofibers with various mass fractions (2, 5, 10 and 20 wt.%) on the mechanical properties of dental resins was investigated. The HApnanofibers were prepared by electrospinning process. The prepared nanofibers were characterized using X-ray diffraction (XRD), scanning electron microscope (SEM), and thermogravimetric analysis (TGA). The average diameter of HApnanofibers was found to be 110 nm. Incorporation of lower loadings of HApnanofibers (2, 5 and 10% wt.%) into the dental resin improved the flexural strength, flexural modulus, compressive strength, and diametral tensile strength of the dental resins; while the higher loading of HAp (20 wt.%) decreased the mechanical properties of dental resins compared with resins containing 10 wt.% HAp.. Therefore, the lower loading of HApnanofibers as novel reinforcing filler can improve the mechanical properties of dental resins.
Keywords
Dental materials; BisGMA; TEGDMA; HApnanofiber; Electrospinning
Download this article as:| Copy the following to cite this article: Shahmirzadi N. J, Inanloo S. H. Effect of Hapnanofibers Prepared by Electrospinning Process on the Mechanical Properties of Dental Resins. Biomed Pharmacol J 2015;8(1) |
| Copy the following to cite this URL: Shahmirzadi N. J, Inanloo S. H. Effect of Hapnanofibers Prepared by Electrospinning Process on the Mechanical Properties of Dental Resins. Biomed Pharmacol J 2015;8(1). Available from: http://biomedpharmajournal.org/?p=1322 |
Introduction
Dental resins have been widely used to restore the teeth since 50 years ago [1]. Despite the significant improvement of dental resins, the main problem of dental resins were deficiencies of 2 mechanical strength and high polymerization shrinkage which responsible for lower lifespan of dental resins [2, 3]. In recent years, the researchers have been widely used from organic polymer matrix (BisGMA, TEGDMA, urethane dimethacrylate (UDMA), etc.), inorganic filler particles, coupling agents, and the initiator–accelerator system for designing of dental composites [4-6]. By development nanotechnology and incorporation of nano scale fillers in the dental resins, the wear-resistant property, esthetic quality, and longevity of dental resin composites have been significantly improved. However, the main problem of dental resins including inadequate mechanical properties of the composites remain.
Various fillers such as calcium phosphates (CaPs) [7], such as hydroxyapatite (HAp) [8, 9], amorphous calcium phosphates (ACP) [10], tetracalcium phosphate (TTCP) [11] and dicalcium phosphate anhydrous (DCPA) [12, 13] have been used to improve the mechanical properties of dental resins. However, the previous studies confirmed that the most nanoparticle fillers did not provide reinforcing effects to significantly improve the mechanical properties of the dental composites. HAp is a natural component of dental enamel and dentine, naturally radiopaque and highly resistant to moisture. HAp has excellent biocompatibility and bioactivity, which is widely used as a material for tissue regeneration [14]. Both nanosized and microsized HAP particles were also studied as dental fillers and the mechanical tests indicated that micro-sized instead of nano-sized HAp was favored in terms of mechanical properties [8, 9]. The aggregation of HAp nanoparticles into the composite dentals decreases the filler dispersion in the composite and consequently, decreases the mechanical properties of the dental resin composites. Therefore, the nanofibers are preferred as reinforced materials compared to particles for HAp nanoparticles. Fong et al. reported that the addition of 5 wt% relative low-strength polymer (Nylon 6) nanofibers could also lead to 36% and 26% increase in flexural strength and modulus, 3 respectively [4]. Furthermore, the addition of nanofibers can reduce the polymerization shrinkage [15]. The nanofibers prepared by eletrospinning process have been widely used for biomedical applications [16-18]. In the electrospinning technique, high voltage is applied between a nozzle and a collector where an electrically charged jet of polymer or composite solution creates. The solvent evaporates before reaching the collector and the nanofibers produce on a collector [19].
The aim of this study was to investigate the reinforcement of BisGMA/TEGDMA dental resins with various mass fractions of HApnanofibers. The HApnanofibers were fabricated by electrospinning process. The structure of electrospunHApnanofibers was determined by scanning electron microscopy (SEM). To evaluate the reinforcing effect of HApnanofibers, the flexural strength (SF), flexural modulus (EY), compressive strength (SC), Vickers micro-hardness (Hm), and diametral tensile strength (DTS) of the dental resins filled with 2, 5, 10, and 20 wt% of HApnanofibers were measured.
Experimental
Materials
BisGMA, TEGDMA, Silica nanoparticles (average size 40 nm), camphorquinone (CQ), ethyl 4-dimethylamino-benzoate (EDMAB), calcium nitrate, sodium dihydrogen phosphate dehydrate, gelatin, urea, and acetone were all purchased from Sigma–Aldrich Company (Sigma, Germany).
Fabrication of HApnanofibers
The HApnanofibers were prepared via electrospinning process. The HAp solution was prepared by mixing calcium nitrate (0.02 mol/L), sodium dihydrogen phosphate dehydrate (0.02 mol/L), gelatin (0.4 g/L), and urea (0.04 mol/L) in an aqueous solution at the room temperature. The prepared solution was loaded into a 5 mL plastic syringe equipped with a syringe needle. This 4 was placed to a KD programmable syringe pump to control the solution flow rate. Then, a high voltage was applied between the needle and the collector and HApnanofibers were produced on the collector. A voltage of 12 kV, with a tip-collector distance of 8 cm, at a speed of 0.5 mL h-1 was applied to the solution and fibers were collected on the cylindrical collector. The schematic of electrospinning process is shown in Fig.1
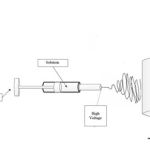 |
Figure 1: The schematic of electrospinning process. |
Preparation of dental resin composites
The resin matrix containing monomers (Bis-GMA/TEGDMA, 49.5/49.5, wt%) and initiators (CQ/4-EDMAB,0.5/0.5, wt%) was uniformly mixed under stirring. Various mass fractions of HAP nanofibers (2, 5, 10 and 20 % wt.) were added into the vial to mix with the dental resins and stirring continued for 12 h. Several drops of acetone were added to reduce the viscosity of mixture. The prepared composite were mixed with the magnetic stir for further 12 h to evaporate the acetone. All the obtained uncured composites were placed in oven under vacuum at room temperature for 8 h to remove air bubbles. After-wards, the composite was added carefully to the rectangular (25 mm × 2 mm × 2 mm) and circle shaped (4 mm × 6 mm and 6 mm × 4 mm) silicon rubber molds covered by glass slides.
Measurement and methods
The powder’s X-ray diffraction (XRD) patterns was recorded at 25 °C on a Philips instrument (X’pertdiffractometer using CuKα radiation) with a scanning speed of 0.03° (2Ө) min-1 to confirm the TiO2 structure. The morphological analyses of the TiO2 nanoparticles was determined using a scanning electron microscopy (SEM, TESCAN, VEGA 3SB).
Weight changes as a function of time and temperature were evaluated during a thermal program from 50 to 600◦C at a heating rate of 10◦C/min in nitrogen atmosphere. The measurement was 5 performed on a thermal gravimetric analyzer (STA409PC, NETZSCH, Germany) using 10 mg of HApnanofibers.
Flexural strength, flexural modulus, compressive strength, and diametral tensile strength of the resin composites were measured using a mechanical testing machine (Texture Technologies, Scarsdale, NY, USA) according to the previous reported methods [20, 21].
Results and Discussion
Characterization of HApnanofibers
The X-ray diffraction pattern of prepared HApnanofibers is illustrated in Fig. 2. As shown, the diffraction of prepared nanofibers indicated the expected peaks for the pure inverse spinel structure of HAp phase (ICDD 09-432). Furthermore, the sharp and narrow diffraction peaks indicated the highly crystalline structure of HApnanofibers.
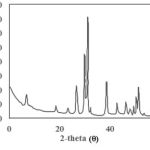 |
Figure 2: XRD pattern of prepared HApnanofibers. |
The SEM image and diameter distribution of the synthesized HApnanofibers is illustrated in Fig. 3. As shown, the fiber diameter distribution of prepared nanofibers was uniform with average diameter of 110 nm.
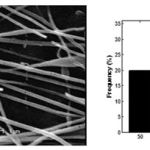 |
Figure 3: SEM image and diameter distribution of the synthesized HApnanofibers. |
Heat treatment was conducted to examine the HAP nanofibers. The results of prepared HApnanofibers heated in atmosphere at different temperatures for 24 h is shown in Fig. 4. After the heat treatment, the 350 ◦C HAp is brown. It can be attributed to a very thin graphite layer on the surface of nanofibers. Nanofibrous samples treated at higher temperatures were white. The graphite layer disappeared due to oxidization at higher temperatures. As shown in the TGA curve (Fig. 4) of HApnanofibers, the weight of HApnanofibrous sample reached the stable stage at about 800 ◦C and the weight loss of HAP nanofibers was about 7.8 wt%. 6
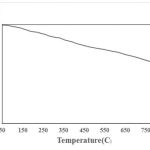 |
Figure 4: The TGA curve of HAP nanofibers. |
Mechanical properties of dental resin composites
Strong mechanical properties of the resin composite are essential for the long-term clinic application of dental restoratives. To evaluate the reinforcing effect of HApnanofibers, flexural strength (SF), flexural modulus (EY), compressive strength (SC),Vickers microhardness (Hm), and diametral tensile strength (DTS) of the dental resins filled with 2, 5, 10 and 20 wt% were measured and the results are shown in Fig. 5. As shown, the addition of HApnanofibers increased the values of SF, EY, SC, Hm, and DTS up to 10%. Further increase in HApnanofibers into the dental resins resulted in the decrease in mechanical properties of dental nanofibrous composites. The values of SF, EY, SC, Hm, and DTS of the unfilled resin were found to be (91.2 ± 2.6) MPa, (2.6 ± 0.2) GPa, (312.32 ± 8.6) MPa, (16.3 ± 0.4) HV, and (29.7 ± 2.5) MPa, respectively. For the dental resin filled with 10 wt% HApnanofibers, the values of SF, EY, SC, Hm, and DTS were found to be (128.5 ± 2.2) MPa, (3.3± 0.2) GPa, (372.3 ± 10.9) MPa, (21.4 ± 0.4) HV and (43.2 ± 1.1) MPa, respectively. These results indicated that 10% HApnanofibers improved the mechanical properties of the dental resin effectively. It was attributed to the strongly embedded HApnanofibers into the matrix. Decrease in mechanical properties of dental resin might be possibly due to the undesirable distribution of HApnanofibers into the matrix.
 |
Figure 5 (a): The flexural strength,(b) flexural modulus, (c) compressive strength, (d) Vickers microhardness, and (e) diametral tensile strength of the dental resins filled withHApnanofibers. |
Morphology of nanofibrous dental composites
The SEM images of fracture surface of dental composites containing HApnanofibers is shown in Fig.6. As shown, the surface of pure dental resin was smooth with oriental fracture lines. The fracture surfaces of HAP nanofibers reinforced resins (Fig. 6b, c) were very rough with no clearly identifiable fracture lines. The HApnanofibers can be uniformly distributed in the dental 7 resins at a lower loading rate (Fig. 6b), which resulted in the improvement on mechanical properties of dental resin. Higher incorporation of HAp into the dental resin produced the more bundles in the dental resin matrix which resulted in decreasing the mechanical properties of dental resins.
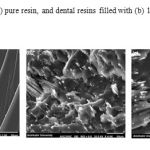 |
Figure 6: SEM images of (a) pure resin, and dental resins filled with (b) 10 and (c) 20 wt. % of HApnanofibers. |
Conclusion
In the present study, the HApnanofibers were successfully fabricated by electospinning process with average diameter of 110 nm. The effect of HApnanofibers on the mechanical properties of Bis-GMA/TEGDMA dental resin was investigated. The results indicated that the flexural strength, flexural modulus, compressive strength, and diametral tensile strength of the resin improved by addition of HApnanofibers up to 10% into the dental resin. Further increase in HApnanofibers due to the undesirable distribution of HApnanofibers into the matrix reduced the mechanical properties of dental resin. Therefore, the novel HApnanofiber can promise as suitable filler for fabricating dental resin composites with improved mechanical properties.
References
- Kim JW, Kim LU, Kim CK, Cho BH, Kim OY. Characteristics of novel dental composites containing2,2-bis[4-(2-methoxy-3-methacryloyloxy propoxy) phenyl]propane as a base resin. Biomacromolecules 2006; 7:154–60.
- Pamela S, Stein JS, Haubenreicb JE, Osborne PB. Composite resin in medicine and dentistry. J Long-Term Effects Med Implants 2005; 15:641–54.
- Stefan Ruttermann SK, Raab WH-M, Janda R. Polymerization shrinkage and hygroscopic expansion of contemporary posterior resin-based filling materials—a comparative study. J Dentistry 2007; 35:806–13. 8
- Fong H. Electrospun nylon 6 nanofiber reinforced Bis-GMA/TEGDMA dental restorative composite resins. Polymer 2004; 45:2427–32.
- Tian M, Gao Y, Liu Y, Liao Y, Hedin NE, Fong H. Fabrication and evaluation of Bis-GMA/TEGDMA dental resins/composites containing nanofibrillar silicate. Dent Mater 2008; 24:235–43.
- Hosseinalipour M, Javadpour J, Rezaie H, Dadras T, Hayati AN. Investigation of mechanical properties of experimentalBis-GMA/TEGDMA dental composite resins containing various mass fractions of silica nanoparticles. J Prosthodont 2010; 19:112–7.
- Mitra SB, Wu D, Holmes BN. An application of nanotechnology in advanced dental materials. J Am Dent Assoc 2003; 134:1382–90.
- Domingo C, Arcis RW, Osorio E, Osorio R, Fanovich MA, Rodriguez-Clemente R, et al. Hydrolytic stability of experimental hydroxyapatite-filled dental composite materials. Dent Mater 2003; 19:478–86.
- Santos C, Clarke RL, Braden M, Guitian F, Davy KW. Water absorption characteristics of dental composites incorporating hydroxyapatite filler. Biomaterials 2002; 23:1897–904.
- Skrtic D, Antonucci JM. Dental composites based on amorphous calcium phosphate – resin composition/physicochemical properties study. J BiomaterAppl 2007; 21:375–93.
- Xu HHK, Weir MD, Sun L. Calcium and phosphate ion releasing composite: effect of pH on release and mechanical properties. Dent Mater 2009; 25:535–42.
- Hockin HK, Xu LS, Weir MD, Takagi S, Chow LC, Hockey B. Effects of incorporating nanosized calcium phosphate particles on properties of whisker-reinforced dental composites. J Biomed Mater Res (ApplBiomater) 2007; 81B:116–25. 9
- Xu HHK, Moreau JL, Sun L, Chow LC. Strength and fluoride release characteristics of a calcium fluoride based dental nanocomposite. Biomaterials 2008; 29:4261–7.
- Domingo C, Arcís RW, López-Macipe A, Osorio R, Rodríguez-Clemente R, Murtra J, Fanovich MA, Toledano M., Dental composites reinforced with hydroxyapatite: Mechanical behavior and absorption/elution characteristics, J Biomed Mater Res. 56(2001) 297-305.
- Emilia J, Anttila OHK, Laurila TK, Lassila LVJ, Vallittu PK, Hernberg RGR. Evaluation of polymerization shrinkage and hydroscopic expansion of fiber-reinforced biocomposites using optical fiber Bragging grating sensors. Dent Mater 2008; 24:1720–7.
- Pham Q. P., Sharma U, Mikos A. G. Electrospinning of Polymeric Nanofibers for Tissue Engineering Applications: A Review. Tissue Eng. 2006; 12:1197-1211.
- Schiffman J.D, Schauer C.L. A Review: Electrospinning of Biopolymer Nanofibers and their Applications, Polymer Rev. 2008; 48: 317-52.
- Zhang Y, Lim C.T , Ramakrishna S, Huang Z.M. Recent development of polymer nanofibers for biomedical and biotechnological applications ,J.Mater. Sci: Mater. Med. 2005; 16: 933-46.
- Huang Z.M, Zhang Y.Z, Kotaki M, Ramakrishna S. A review on polymer nanofiber by electrospinning and their applications in nanocomposites, Compos.Sci. Technol. 2003; 63:2223–53.
- Liu F, Wang R, Cheng Y, Jiang X, Zhang Q, Zhu M. Polymer grafted hydroxyapatite whisker as a filler for dental composite resin with enhanced physical and mechanical properties. Mater SciEng C 2013; 33:4994–5000.
- Liu J, Li K, Wang H, Zhu M, Xu H, Yan H. Self-assembly of hydroxyapatite nanostructures by microwave irradiation. Nanotechnology 2005; 16:82–7.







