Manuscript accepted on :
Published online on: 17-11-2015
Plagiarism Check: Yes
Faham Khamesipour1, Seyed Mohammadreza Hashemian2*, Ali Akbar Velayati2 and Payam Tabarsi3
1Young Researchers and Elite Club, Shahrekord Branch, Islamic Azad University, Shahrekord, Iran. 2Clinical Tuberculosis and Epidemiology Research Center, National Research Institute of Tuberculosis and Lung Diseases (NRITLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran. 3National Research Institute of Tuberculosis and Lung Diseases, Masih Daneshvari Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran. Correspondence author: E-mail:Dr_Faham@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/641
Abstract
Teicoplanin is an antibiotic used in the prevention and treatment of serious infections caused by gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA) and Enterococcusfaecalis. It is a semi-synthetic glycopeptide antibiotic with a spectrum of activities similar to vancomycin. Its mechanism of action is inhibition of bacterial cell wall synthesis:Teicoplanin is marketed by Sanofi Aventis Coperation under the brand name of Targocid. It has been shown that oral administration of teicoplanin is effective in the treatment of Pseudomembranous colitis and Clostridium difficile-associated diarrhoea, with comparable efficacy to vancomycin.The effectiveness of this antibiotic is associated with its carbon chain length.It’s tried in this review article to introduce teicoplanin synthases, structure and its structure effect on treatment and also introduce the advantages of teicoplanin in bacterial infection treatment and compared its effects with some other antibiotics like vancomycin and linezolid.Based on the above data, it can be concluded that Teicoplanin usage, specially intervenes injection of it, is a successful antibiotic treatment against bacterial infections caused by Gram-positive bacteria, particularly methicillin-resistant Staphylococcus aurous (MRSA). The teicoplanin effect is directly related to the length of its carbon chain. It was shown that treatment with combination of teicoplanin and vancomycin or teicoplanin and linezolid have more influence over the treatment process. The most important advantage of teicoplanin usage in treatments is its lower side effects on patients than other antibiotics.
Keywords
carbon chain; gram-positive; methicillin-resistant Staphylococcus aureus (MRSA); Teicoplanin
Download this article as:| Copy the following to cite this article: Khamesipour F, Hashemian S. M, Tabarsi P, Velayati A. A. A Review of Teicoplanin Used in the Prevention and Treatment of Serious Infections Caused by Gram-Positive Bacteria and Compared Its Effects with Some other Antibiotics. Biomed Pharmacol J 2015;8(1) |
| Copy the following to cite this URL: Khamesipour F, Hashemian S. M, Tabarsi P, Velayati A. A. A Review of Teicoplanin Used in the Prevention and Treatment of Serious Infections Caused by Gram-Positive Bacteria and Compared Its Effects with Some other Antibiotics. Biomed Pharmacol J 2015;8(1). Available from: http://biomedpharmajournal.org/?p=818 |
Introduction
Chemical Structure
In fact,Teicoplanin is composed of several chemicals,5large parts called teicoplanin A2-1 through A2-5 and 4 small parts called teicoplanin RS-1 through RS-4 and has aglycopeptide core called teicoplanin A3-1[1].This ring binds to mannose and N-acetyl glucosamine[2].The major and minor components also contain a third carbohydrate moiety-β-D-glucosamine and differ only by the length and conformation of a side chain attached to it[3, 4]. The overall structure of this compound can be seen in Figure 1.
Teicoplanin Biosynthesis
Teicoplanin refers to a set of natural products isolated from the fermentation broth of a strain of Actinoplanesteichomyceticus, consisting of five subcategories[5]. These subcategories possess a common aglycone, or core, composed of seven amino acids bound by peptide and ether bonds to form a four-ring system and differ by the fatty acyl side-chain attached to the sugar[6]. The origin of these seven amino acids in teicoplanin biosynthesis was studied by 1H and 13C nuclear magnetic resonance. The resultsshow that amino acids AA1, AA2, AA4, AA5, and AA6 are derived from tyrosine, and amino acids AA3 and AA7 are derived from acetate. Specifically, teicoplanin contains 4-hydroxyphenylglycine and 3,5-dihydroxyphenylglycine residues, a chlorine atom attached on each of the tyrosine residues, and three sugar moieties, including N-fatty acyl-β-D-glucosamine, N-acetyl-β-D-glucosamine, and D-mannose[7].
Gene Cluster
The investigation of teicoplanin biosynthesis gene cluster shows OFR 49 in the path of biosynthesis, transfer, resistance, and regulation of gene expression[8, 9].OFR 35 identified in this path is similar to gene clusters relevant to in other glycopeptide genes. The function of each of these genes is described by Li and co-workers[10].
Heptapeptide Backbone Synthesis
Analysis indicated tyrosine and three types of non-proteinogenic amino acids, (S)-4-hydroxyphenylglycine, 3,5-dihydroxyphenylglycine, and β-hydroxytyrosine as the glycopeptidebuilding blocks of teicoplanin [11]. In total, six of the seven total amino acids composing teicoplanin backbone are composed of nonproteinogenic or modified amino acids[12]. Cooperation and activity of eleven enzymes are responsible for preparation and synthesis of these six residues. Teicoplanin contains two chlorinated positions, 2 (3-Cl-Tyr) and 6 (3-Cl-β-Hty) [6, 10, 13]. Halogenase ORF8* has been proposed to have a role in catalyzing the halogenation process of these amino acids[14]. It seems that chlorination process occurs at early stages in Teicoplanin biosynthesis and prior to phenolic oxidative coupling, with the possibility of tyrosine or β-hydroxytyrosine being the substrate of chlorination[15].The biosynthesis of heptapeptideroot is performed by four nonribosomal peptide synthetases called TeiA, TeiB, TeiC, and TeiD[5, 16]. Each module has a domain for amino acid selection and activation through aminoacyl-AMP. The catalytic domain in modules 3 and 4 of non-ribosomal peptide synthetase are linearly and activated by (S)-4-hydroxyphenylglycine and 3, 5-dihydroxyphenylglycine. In addition to these modules for amino acid selection and activation, each module has a thiolation domain modified with phosphopantetheine to provide a thiol for covalent aminoacyl-S-enzyme formation[17, 18].
Modification After Heptapeptide Backbone Formation
Once the heptapeptide backbone has been formed and synthesized, the process of catalyzing the linear structure is begun[19].Studies on gene disruption indicate that cytochrome P450 oxygenaseis an enzyme performing the coupling reactions. OxyB has been suggested to form the first ring with coupling residues 4 and 6. Then, OxyA couples residues 2 and 4, followed by the formation of a C-C bond between residues 5 and 7 by OxyC. Fourth enzyme catalyzes the coupling of residues 1 and 3 that for this purpose,OxyB/OxyA/OxyCplay a role[19, 20].
The process of specific glycosylation occurs after the formation of the heptpeptideaglycone[21]. Given to the data collected for glycosylation of the teicoplanin aglycone it is shown that three separate glycosyltransferases are required[22]. Two of these glycosyltransferases are involved in the addition of the N-fatty acyl-β-D-glucosamine and N-acetyl-β-D-glucosamine units. The third enzyme, which is a mannosyltransferase, is responsible for the addition of the D-mannose unit onto residue 7. The fatty acyl chain is connected by amide bond to the glucosamine moiety by the action of an acyl transferase. In addition to glycosylation, some genes have been suggested to code for deacetylases[23, 24]
Summary of Antibacterial Activity of Teicoplanin
Previous studies have indicated high inhibitory activity of Teicoplanin against Staphylococcus aureus, as well asthoseresistant tomethicillin andoxacillin[25, 26].The general similarity of teicoplanin and vancomycin are also shown. All Streptococci are sensitive to Teicoplanin[27], although the relative susceptibility of coagulase-negative staphylococci to teicoplanin and vancomyc in varied. Studies have shown that Clostridium species such as C. diffilce, C.perjringense, Peptostreptococcus species, Propionibacteriumacens, Corynebacteriumjeikeium and resistant species of Corynebacterium group D2are sensitive to low concentrations of Teicoplanin[28, 29].
The minimum bactericidal concentration(MBC) forteicoplanin is usually less than or equal to 2 dilutions higher than the minimum inhibitory concentration 90% (MIC90)for Streptococcuspneumoniae, S. aureus, S.epidermidis and in some studies for some samples of coagulase-negative staphylococci[25, 30]. The in vitrobactericidalactionfor the teicoplanin, similar tovancomycin, is slow.Teicoplanin in combination with aminoglycosides shows synergistic inhibitory activity against most bacteria ofS. aureus, coagulase negative staphylococci and enterococci.Susceptibility of E. faecium to various antibiotics has been shown a significant increase in resistance to penicillin Gandgentamicin, but susceptibility to teicoplanin and vancomycin is stable. In animal models of gram-positive endocarditis, teicoplanin and vancomycin similarly, reduction of bacterial titers in cardiac valvularvegetations was examined a few hours after drug administration, but 10 days after treatment, higher percentage of vegetation by teicoplanin was free of contamination[31-33].
Introducing The Pharmacokinetic Properties Of Teicoplanin
Result of injecting 6 mg/kg teicoplanin in the mean peak serum concentration at 30 minutes and 4 hours after intravenous and in tramuscular injection has been reported 43 and 12 mg/l, respectively. Insteady state, the concentration of intravenous teicoplanin after intravenouslyinjecting 6 and 12 mg/kg after 12 hours has been reported 14 and 23 mg/l, respectively. And the same results were obtained after 24 hours. Teicoplanin absorption rate after intramuscular administration is equivalent to the rate after intravenous injection. It seems that injecting a dose of 15 mg/kg is needed after a dose of 8mg/kgin day thereafter to maintain the concentration above 10 mg/l of teicoplanin in neonates[34].
Apparent volume of distribution at steady state after intravenous injection of 6 to 15mg/kg teicoplanin was approximately0.8 to 1.6kg/l that was higher than reports in previous studies in which serum samples were collected for a short period. The average concentration of teicoplanin in atrial appendage was 8 / 2-7 / 3 times the average concentration simultaneously obtained in serum, and the highest concentrations in the heart and pericardium tissue was 4 hours after an intravenous dose of 800 mg. Penetration into the cerebrospinal fluid is minimal after in travenous administration, but drug concentrations in the cerebrospinal fluid is reported more than 40 milligrams per liter after intravenous administration of teicoplanin at a dose of 20mg per 24 or48 hours[35, 36].
Body metabolism rate for teicoplanin is slight (about 3%). Total body clearance of teicoplanin after intravenous administration of3 to30 mg/kgin healthy volunteers is in the range 10-13 ml/h/kg. Renal clearance is 8 to 12 ml/h/kg which implies that it is almost eliminated by renalmechanismsare.On average, studies in which the duration of sample collection is 3 weeks after the last dose have shown that elimination half-life of teicoplanin will be 155-168 h after intravenous administration and 182 hours after the intramuscular injection.
Total body and renalclearanceratesforteicoplanin correlates with creatinine clearance and reduces inpatients with impaired renal function. Teicoplanin cannot be removed by hemodialysis cycle, regardless of the dialysis membrane[37, 38].
Inpatients with a history of intravenous drug use treated with teicoplanin for bacterial endocarditis, the average amount of total body andrenal clearanceis reported higher and more diversified than that in healthy subjects and elimination half-life is reduced[39, 40].
An Example of Teicoplanin Treatment Effects
Studies relevant to the teicoplanin effects in treating bacteraemia and intravascular infections in patients without neutropenia stay largely non-comparative. Comparative investigations have shown that daily administration of 6 mg/kg teicoplanin has the same effect with12-hour administration of 15 mg/kg vancomycin. Daily use of only 400-800 mg teicoplanin cures 84 to 93% of patients with bacteraemia caused mostly byS.aureus clinically and bacteriologically and is successful in 90 to 100% of patients with streptococcalorenterococcalendocarditis[41].
Recovery is obtained in 89 to 100% of patients with skin and soft-tissue infections (caused mainly by S. aureusorS.epidermidis) treated with 200 to 800 mg teicoplanin once a day intravenouslyor intramuscularly [42, 43].
Non-comparative tests of teicoplanin (usually 6 mg/kg once daily) in patients with acute/ chronic Osteomyelitis or Septic Arthritis caused by gram-positive bacteria, have led to clinical cure in 83 to 100% of patients[44, 45].
A combination of teicoplanin and ciprofloxacin is significantly more effective than either ciprofloxacin or ceftriaxone alone in relieving respiratory tract infections. In non-comparative studies, teicoplaninalone results in clinical cure or improvement in approximately 91% of patients with respiratory tract infections. In the treatment of Clostridium difficile resulting in severe diarrhea and colitis, oral administration of 100 mg teicoplanin twice a day and 500 mg vancomycin four times a day can improve 96% and 100% of patients, respectively[46-48].
Once teicoplanin is used as the initial treatment in patients with cancer and neutropenia, its effect is similar to that of vancomycin. Teicoplanin was associated with a more tolerant with a lower incidence of super infection caused by Candida species, indicating that teicoplanin is a viable alternative to vancomycin. Adding teicoplanin to piperacillinplusamikacin has no clinical effectiveness. Controversy still remains over the need for these drugs, from drugs election and timing for the introduction of antibiotics to experimental diets that all depends on environmental conditions[49-51].
While no improvement was observed in patients, they were prescribed with teicoplanin, and positive results were shown in patients with gram-positive bacterial infection.Teicoplanin was effective for secondary treatment of patients who had a negative response to experimental treatment with either ceftazidime alone or in combination with amikacin, piperacillinplusamikacin, or one of the cephalosporiumandaminoglycoside antibiotics[52, 53].
Inpatients undergoing hip and knee arthroplasty, only intravenous dose of 400 mg teicoplanin has a similar effectiveness with 4 post-surgery doses of cefamandole or 5 post-surgery doses of cefazolinin anesthesia induction. Clinical experience with teicoplaninis limited in infants and children. However, preliminary data in children with sepsis, upper and lower respiratory tract infection, skin or soft tissue infection and in febrile children with neutropenia shows that 6 to10mg/kg teicoplanin once a day is effective in the treatment of gram-positive infections[54-56].
As a result of the efficiency of teicoplanin against gram-positive infections, once-daily intravenous orintramuscular injection of teicoplanin is suitable for out patients. After the success ofteicoplanin in outpatients or patients at first hospitalization and thereafter, it showed success in cares after discharge. Teicoplanin has been successfully used to treat skin and soft tissue infection, bone and joint infection, and mediastinitisaftercoronary artery bypass surgery[57].
Comparing the Effect of Teicoplanin and Linezolid
Linezolid is the only commercially available oxazolidinone using for gram-positive infections, although, a few papers are published particularly on its use in acute illnesses [58, 59]. Therefore, a prospective randomized study was conducted to compare linezolid with glycopeptide antibiotic, teicoplanin, for suspicious or proven treatment of gram-positive infections in intensive care unit population by Cepedaet al.[58].
In this regard, a perspective double-blind double-dummy study was designed. The patients were randomly divided into two groups: A) patients who received intravenous linezolid(600 mg /12hours) plus intravenous placebo-teicoplanin (one doses every 12hours and after three injections, one doseper24hours). B) Patients who received teicoplanin(3 injections per 12 hours at a dose of 400 mg and then one injection per 12 hours) plus placebo-linezolid (one dose per 12 hours and after three injections, one doseper 24 hours). Other antibiotics were used in combination with testing drugs in experimental treatment. Clinical and microbiological evaluation of the first week was done daily and then, in day 8 and 21.
In this study, 100 patients received linezolid plus placebo-teicoplanin and 102 patients received teicoplanin plus placebo-linezolid. Figures obtained from population were similar in both groups. At the end of treatment, clinical success [71 (78.9%) linezolid versus 67 (72.8%) teicoplanin] and microbiological success [49 (70.0%) versus 45 (66 .2%)] were similar. Side effects and the success rate of short-term and long-term follow-up were also the same. Linezolid was superior in the initial distance against the colonization of methicillin-resistant Staphylococcusaureus (end of treatment, 51.1% versus 18.6%, P = 0.002). The results of researches indicated two MRSA samples less sensitive toteicoplanin. The results of this study showed that linezolid has a similar effectiveness with teicoplanin in the treatment of infections caused by gram-positive bacteria. The difference is that MRSA short-term clearance achieved by linezolid represents its better penetration in to the skin and mucosa[59].
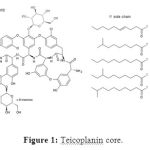 |
Figure 1: Teicoplanin core. |
Comparing the Effect of Teicoplanin and Vancomycin
Teicoplanin and vancomycin are two commonly used treatments forgram-positive bacterial infections, especially methicillin-resistant Staphylococcusaureus (MRSA)[60, 61]. There is an uncertainty about the effects ofteicoplanin compared with vancomycin in their effectiveness on renal function. Some previous studies have shown that teicoplaninislessnephrotoxicthanvancomycin. To evaluate the efficacy and safety of vancomycin in comparison with teicoplanin in patients with proven or suspected infection, Cavalcanti et al., searched articles published in different languages in association with comparing the efficacy of teicoplanin and vancomycin. They evaluated independently methodological quality and data extracted using a standardized data extraction form in various articles. They gathered information from 24 independent studies and concluded that teicoplanin reduces the risk of nephrotoxicity compared with vancomycin (risk ratio 0.66, 95% confidence interval 0.48 to 90).They reported the effect of teicoplanin and vancomycin the same for clinical improvement (RR 1.03, 95% CI 0.98 to 1.08), microbiological cure (RR 0.98, 95% CI 0.93 to 1.03) and mortality(RR 1.02, 95% CI 0.79 to 1.30). Side effects including skin rash (RR 0.57, 95% CI 0.35 to 0.92),red man syndrome(RR 0.21, 95% CI 0.08 to 0.59)and total side effects (RR 0.73, 95% CI 0.53 to 1.00) resulted from administrating teicoplanin compared to vancomycin were observed less. The risk of nephrotoxicity with teicoplaninin patients with (RR 0.51, 95% CI 0.30 to 0.88)or with out(RR 0.31, 95% 0.07 to 1.50)aminoglycosides has been less.
Finally and generally, these studies showed that, teicoplanin and vancomycin are effective in treating patients with provenor suspected infection. Yet, incidence of adverse effects, including nephrotoxicity with teicoplanin administration will be lower. Since the group was not able to assess patients with acute kidney injury requiring dialysis, it did not become clear that different effects on kidney function should be under the impression of what antibiotic is prescribed.Although it seems logical that teicoplanin to be prescribed for patients with a high risk of AKI requiring dialysis due to the lower risk of nephrotoxicity resulted from antibiotic usage[31].
Based on the above data, it can be concluded that Teicoplanin usage, specially intervenes injection of it, is a successful antibiotic treatment against bacterial infections caused by Gram-positive bacteria, particularly methicillin-resistant Staphylococcus aurous (MRSA).The teicoplanin effect is directly related to the length of its carbon chain. It was shown that treatment with combination of teicoplanin and vancomycin or teicoplanin and linezolid have more influence over the treatment process. The most important advantage of teicoplanin usage in treatments is its lower side effects on patients than other antibiotics.
Reference
- Barna JC, Williams DH, Stone DJ, Leung TC, Doddrell DM. Structure elucidation of the teicoplanin antibiotics.J AmChem Soc.vol. 1984; 106 (17):4895-4902.
- Hunt AH, Molloy RM, Occolowitz JL, Marconi GG, Debono M. Structure of themajor glycopeptide of the teicoplanin complex.J Am Chem Soc.1984; 106(17):4891-4895.
- Parenti F. Structure and mechanism of action of teicoplanin.J Hosp Infect.1986; 7:79-83.
- Bick MJ, Banik JJ, Darst SA,BradyCrystal structures of the glycopeptide sulfotransferase Teg12 in a complex with the teicoplanin aglycone.Biochem.2010; 49(19): 4159-4168.
- Jung HM, Jeya M, Kim SY, Moon HJ, Singh RK, Zhang WY, Lee JK. Biosynthesis, biotechnological production, and application of teicoplanin: current state and perspectives.Appl Microbiol Biotechnol.2009; 84(3):417-428.
- Cryle MJ, Staaden J,Schlichting I. Structural characterization of CYP165D3, a cytochrome P450 involved in phenolic coupling in teicoplanin biosynthesis.Arch Biochem Biophys.2011; 507(1):163-173.
- Heydorn A, Petersen BO, Duus JO, Bergmann S, Suhr-Jessen T, Nielsen J. Biosynthetic Studies of the Glycopeptide Teicoplanin by 1H and 13C NMR.J Biol Chem.2000; 275(9):6201-6206.
- Sosio M, Kloosterman H, Bianchi A, de Vreugd P, Dijkhuizen L, Donadio S.Organization of the teicoplanin gene cluster in Actinoplanes teichomyceticus.Microbiol.2004; 150(1):95-102.
- Chan HC, Huang YT, Lyu SY, Huang CJ, Li YS, Liu YC, Chou CC, Tsai MD, Li TL. Regioselective deacetylation based on teicoplanin-complexed Orf2* crystal structures.Mol Biosyst.2011; 7(4):1224-1231.
- Li TL, Huang F, Haydock SF, Mironenko T, LeadlayPF, Spencer JB. Biosynthetic gene cluster of the glycopeptide antibiotic teicoplanin: characterization of two glycosyltransferases and the key acyltransferase.Chem Biol.2004; 11(1):107-119.
- Kahne D, LeimkuhlerC, Lu W, Walsh C. Glycopeptide andlipoglycopeptide antibiotics.Chem Rev.2005; 105(2):425-448.
- Recktenwald J, Shawky R, Puk O, Pfennig F, Keller U, Wohlleben W, Pelzer S. Nonribosomal biosynthesis of vancomycin-type antibiotics: a heptapeptide backbone and eight peptide synthetase modules.Microbiol.2002; 148(4):1105-1118.
- Malabarba A, Ciabatti R, Kettenring J, Ferrari P, Scotti R, Goldstein BP, Denaro M. Amides of de-acetylglucosaminyl-deoxy teicoplanin active against highly glycopeptide-resistant enterococci. Synthesis and antibacterial activity.J Antibiot.1994; 47(12):1493-1506.
- Sosio M, Bianchi A, Bossi E, Donadio S. Teicoplanin biosynthesis genes in Actinoplanes teichomyceticus.Antonie Van Leeuwenhoek.2000; 78(3-4):379-384.
- Li Z, Rupasinghe SG, Schuler MA, Nair SK. Crystal structure of a phenol‐coupling P450 monooxygenase involved in teicoplanin biosynthesis.Proteins: Structure, Function, and Bioinformatics.2011; 79(6):1728-1738.
- Horbal L, Kobylyanskyy A, Truman AW, Zaburranyi N, Ostash B, Luzhetskyy A, Marinelli F, Fedorenko V. The pathway-specific regulatory genes, tei15* and tei16*, are the master switches of teicoplanin production in Actinoplanes teichomyceticus.Appl Microbiol Biotechnol.2014; 1-15.
- Donadio S, Sosio M, Stegmann E, Weber T, Wohlleben W. Comparative analysis and insights into the evolution of gene clusters for glycopeptide antibiotic biosynthesis.Mol Genet Genomics.2005; 274(1):40-50.
- Malabarba A, Trani A, Strazzolini P, CiettoG,Ferrari P, Tarzia G, Pallanza R, Berti M. Synthesis and biological properties of N63-carboxamides of teicoplanin antibiotics. Structure-activity relationships.J Med Chem.1989; 32(11):2450-2460.
- Hadatsch B, Butz D, SchmiedererT,Steudle J, Wohlleben W, Süssmuth R, Stegmann E. The biosynthesis of teicoplanin-type glycopeptide antibiotics: assignment of p450 mono-oxygenases to side chain cyclizations of glycopeptide a47934.ChemBiol.2007; 14(9):1078-1089.
- Wu J, Su P, Huang J, Wang S, Yang Y. Synthesis of teicoplanin-modified hybrid magnetic mesoporous silica nanoparticles and their application in chiral separation of racemic compounds. J Colloid Interf Sci.2013; 399: 107-114.
- Kaplan J, Korty BD, Axelsen PH, Loll PJ.The role of sugar residues in molecular recognition by vancomycin.J Med Chem.2001; 44(11):1837-1840.
- Pathak TP, Miller SJ. Chemical tailoring of teicoplanin with site-selective reactions.J Am Chem Soc.2013; 135(22):8415-8422.
- Gasper MP, Berthod A, Nair UB, Armstrong DW. Comparison and modeling study of vancomycin, ristocetin A, and teicoplanin for CE enantioseparations.Anal Chem.1996; 68 (15):2501-2514.
- Guillaume Y, Truong T, Millet J, Nicod L, Guinchard C, Robert J,Thomassin M. Chiral discrimination of phenoxypropionic acid herbicides on teicoplanin phase: Effect of mobile phase modifier.Chromatographia.2002; 55(3-4): 143-148.
- Traczewski MM, Katz BD, Steenbergen JN, Brown SD. Inhibitory and bactericidal activities of daptomycin, vancomycin, and teicoplanin against methicillin-resistant Staphylococcus aureus isolates collected from 1985 to 2007.Antimicrob Agents Chemother.2009; 53(5):1735-1738.
- Tsuchiya H, Sato M, Miyazaki T, Fujiwara S, Tanigaki S, Ohyama M, Tanaka T, Iinuma M. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant< i> Staphylococcus aureus</i>.J Ethnopharmacol.1996; 50(1):27-34.
- Appelbaum P, Spangler S, Crotty E, Jacobs M. Susceptibility of penicillin-sensitive and-resistant strains of Streptococcus pneumoniae to new antimicrobial agents, including daptomycin, teicoplanin, cefpodoxime and quinolones.J Antimicrob Chemother.1989; 23(4):509-516.
- Stinear TP, Olden DC, Johnson PD, Davies JK, Grayson ML.Enterococcal< i> vanB</i> resistance locus in anaerobic bacteria in human faeces.The Lancet.2001; 357(9259): 855-856.
- Vanderhofstadt M, André M, Lonchay C, Levecque P, Holemans X,Canon JL,D’Hondt L.< i> Clostridium tertium</i> bacteremia: contamination or true pathogen? A report of two cases and a review of the literature.Int J Infect Dis.2010; 14: e335-e337.
- Nailor MD, Sobel JD. Antibiotics for gram-positive bacterial infections: vancomycin, teicoplanin, quinupristin/dalfopristin, oxazolidinones, daptomycin, dalbavancin, and telavancin.Infect Dis Clin North Am.2009; 23(4): 965-982.
- Cavalcanti AB, Goncalves AR, Almeida CS, Bugano D, Silva E. Teicoplanin versus vancomycin for proven or suspected infection.Cochrane Database Syst Rev.2010; 6.
- Cremniter J, Slassi A, Quincampoix JC, Sivadon-Tardy V, Bauer T, Porcher R, Lortat-Jacob A, Piriou P, Judet T, Herrmann JL. Decreased susceptibility to teicoplanin and vancomycin in coagulase-negative staphylococci isolated from orthopedic-device-associated infections.J Clin Microbiol.2010; 48(4): 1428-1431.
- SvetitskyS,Leibovici L, Paul C. Comparative efficacy and safety of vancomycin versus teicoplanin: systematic review and meta-analysis.Antimicrob Agents Chemother.2009; 53(10): 4069-4079.
- Niwa T, Imanishi Y, Ohmori T, Matsuura K, Murakami N, Itoh Y. Significance of individual adjustment of initial loading dosage of teicoplanin based on population pharmacokinetics.Int J Antimicrob Agents.2010; 35(5):507-510.
- Brink A, Richards G, Cummins R, Lambson J. Recommendations to achieve rapid therapeutic teicoplanin plasma concentrations in adult hospitalised patients treated for sepsis.Int J Antimicrob Agents.2008; 32(5):455-458.
- Brogden RN, Peters DH. Teicoplanin.Drugs. 1994;47(5): 823-854.
- Verbist L, Tjandramaga B, Hendrickx B, Van Hecken A, Van Melle P, Verbesselt R, Verhaegen J, De Schepper In vitro activity and human pharmacokinetics of teicoplanin.Antimicrob Agents Chemother.1984; 26(6): 881-886.
- Wilson APR. Clinical pharmacokinetics of teicoplanin.Clin Pharmacokinet.2000; 39(3): 167-183.
- Habib G, Hoen B, Tornos P, Thuny F, Prendergast B, Vilacosta I, Moreillon P, de Jesus Antunes M, Thilen U, Lekakis J. Guidelines on the prevention, diagnosis, and treatment of infectiveendocarditis (new version 2009) The Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC).Eur Heart J.2009; ehp285.
- Rybak MJ, Lerner S, Levine D, Albrecht L, McNeilP,Thompson G, Kenny M, Yuh L.Teicoplanin pharmacokinetics in intravenous drug abusers being treated for bacterial endocarditis.Antimicrob Agents Chemother.1991; 35(4):696-700.
- Menichetti F, Martino P, Bucaneve G, Gentile G, D’Antonio D, Liso V, Ricci P, Nosari AM, Buelli M, Carotenuto M. Effects of teicoplanin and those of vancomycin in initial empirical antibiotic regimen for febrile, neutropenic patients with hematologic malignancies. Gimema Infection Program.Antimicrob Agents Chemother.1994; 38(9):2041-2046.
- Doern GV, Jones RN, Pfaller MA, Kugler KC, Beach ML. Bacterial pathogens isolated from patients with skin and soft tissue infections: frequency of occurrence and antimicrobial susceptibility patterns from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 1997).Diagn Microbiol Infect Dis.1999; 34(1):65-72.
- Logman JFS, Stephens J, Heeg B, Haider S, Cappelleri J, Nathwani D, Tice A, van Hout BA. Comparative effectiveness of antibiotics for the treatment of MRSA complicated skin and soft tissue infections.Curr Med Res Opin.2010; 26(7):1565-1578.
- Glupczynski Y, Lagast H, Van Der Auwera P, Thys J, Crokaert F, Yourassowsky E, Meunier-Carpentier F, Klastersky J, Kains J, Serruys-Schoutens E. Clinical evaluation of teicoplanin for therapy of severe infections caused by gram-positive bacteria.Antimicrob Agents Chemother. 1986; 29(1):52-57.
- Mathews CJ, Weston VC, Jones A, Field M, Coakley G. Bacterial septic arthritis in adults.The Lancet.2010; 375(9717):846-855.
- Kelsey S, Weinhardt B, Collins P, Newland A. Teicoplanin plus ciprofloxacin versus gentamicin plus piperacillin in the treatment of febrile neutropenic patients.Eur J Clin Microbiol.1992; 11(6): 509-514.
- Kelsey SM, Collins PW, Delord C, Weinhard B, Newland AC. A randomized study of teicoplanin plus ciprofloxacin versus gentamicin plus piperacillin for the empirical treatment of fever inneutropenic patients.Br J Haematol.1990; 76(s2): 10-13.
- Van Der Auwera P, Joly P. Comparative in-vitro activities of teicoplanin, vancomycin, coumermycin and ciprofloxacin, alone and in combination with rifampicin or LM427, against Staphylococcus aureus.J Antimicrob Chemother.1987; 19(3): 313-320.
- De Naurois J, Novitzky-Basso I, Gill M, Marti FM, Cullen M, Roila F. Management of febrile neutropenia: ESMO clinical practice guidelines.Ann Oncol.2010; 21(suppl 5): v252-v256.
- Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T, Feld R, Pizzo PA, Rolston KV, Shenep JL. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer.Clin Infect Dis.2002; 34(6):730-751.
- Pizzo PA, Hathorn JW, Hiemenz J, Browne M, Commers J, Cotton D, Gress J, Longo D, Marshall D, McKnight J. A randomized trial comparing ceftazidime alone with combination antibiotic therapy in cancer patients with fever and neutropenia. N Engl J Med.1986; 315(9): 552-558.
- Cometta A, Calandra T, Gaya H, Zinner SH, De Bock R, Del Favero A, Bucaneve G, Crokaert F, Kern WV, Klastersky J. Monotherapy with meropenem versus combination therapy with ceftazidime plus amikacin asempiric therapy for fever in granulocytopenic patients with cancer. The International Antimicrobial Therapy Cooperative Group of the European Organization for Research and Treatment of Cancer and the Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto Infection Program.Antimicrob Agents Chemother.1996; 40(5): 1108-1115.
- Drago L, De Vecchi E, Nicola L, Legnani D, Lombardi A, Gismondo. In vitro synergy and selection of resistance by fluoroquinolones plus amikacin or β-lactams against extended-spectrum β-lactamase-producing Escherichia coli.In Vitro.2013; 17(1).
- Kanellakopoulou K, Papadopoulos A, Varvaroussis D, Varvaroussis A, Giamarellos-Bourboulis EG, Pagonas A, Stergiou A, Papadelis P, Nikolaidis V, Giamarellou H.Efficacy of teicoplanin for the prevention of surgical site infections after total hip or knee arthroplasty: a prospective, open-label study.Int J Antimicrob Agents.2009; 33(5): 437-440.
- Lazzarini L, Novelli A, Marzano N, Timillero L, Fallani S, Viola R, de Lalla F. Regional and systemic prophylaxis with teicoplanin in total knee arthroplasty.J Arthroplasty.2003; 18(3): 342-346.
- Suter F, Avai A, Fusco U, Gerundini M, Caprioli S, Maggiolo F. Teicoplanin versus cefamandole in the prevention of infection in total hip replacement.Eur J Clin Microbiol.1994; 13(10): 793-796.
- Wilson A, Taylor B, Treasure T, Grüneberg R, Patton K, Felmingham D, Sturridge S. Antibiotic prophylaxis in cardiac surgery: a prospective comparison of two dosage regimens of teicoplanin with a combination of flucloxacillin and tobramycin.J Antimicrob Chemother.1988; 21(2): 213-223.
- Cepeda JA, Whitehouse T, Cooper B, Hails J, Jones K, Kwaku F,Taylor L, Hayman S, Shaw S, Kibbler C. Linezolid versus teicoplanin in the treatment of Gram-positive infections in the critically ill: a randomized, double-blind, multicentre study.J Antimicrob Chemother.2004; 53(2): 345-355.
- Whitehouse T, Cepeda JA, Shulman R, Aarons L, Nalda-Molina R, Tobin C, MacGowan A, Shaw S, KibblerC, Singer M. Pharmacokinetic studies of linezolid and teicoplanin in the critically ill.J Antimicrob Chemother.2005; 55(3): 333-340.
- Hiramatsu K.“ Vancomycin resistance in staphylococci.Drug Resist Update.1998; 1(2): 135-150.
- Kullar R, Davis SL, Levine DP, Rybak MJ. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets.Clin Infect Dis.2011; 52(8):975-981.







