Manuscript accepted on :March 10, 2015
Published online on: 05-12-2015
Plagiarism Check: Yes
Leila Vahabi1, Ramesh Monajemi2, Seyed Ahmad Hosseini3*
1Department of Biochemistry, Falavarjan Branch, Islamic Azad University, Isfahan, Iran. 2Department of Biology, Falavarjan Branch, Islamic Azad University, Isfahan, Iran. 3Nutrition and Metabolic Diseases Research Center ,Ahvaz Jundishapur, University of Medical Sciences, Ahvaz, Iran *Corresponding Author: Email: seyedahmadhosseini@yahoo.com
Abstract
For years the plants as a natural resource to be used for the treatment of cancer. But if the development of anticancer drugs from natural sources such as plants needs to screening tests. This study aimed to assess the cytotoxic effect of Pyracantha coccinea fruit methanol extract on Hela cell line and evaluation of antioxidant properties and total phenolic content of methanol and aqueous extract of Pyracantha coccinea fruit was done. Hela cell culture medium RPMI-1640 containing 10% fetal bovine serum were cultured with 5% CO2. Fruit of Pyracantha coccinea, after being dried, solvent and aqueous was extracted with methanol by maceration method. Cytotoxic effect of the extract in different concentrations was evaluated by MTT assay at 72 hours. Pyracantha coccinea fruit was collected from Esfahan. Aqueous and methanol extracts preparation with Soaking method. Antioxidant activity was determined by scavenging of DPPH assay. Also this Pyracantha coccinea fruit extracts were analyzed for total phenolic contents with Folin-Ciocalteu reagent. Gallic acid was used as a standard compound and the total phenols were expressed as mg gallic acid /g dry extract equivalents. the cytotoxicity of the methanol extract at a concentration of 800 µg/ml was higher than other concentrations. The total phenolic content and antioxidant properties of the methanol extract was higher than Aqueous extract. The results indicate a weak cytotoxic effects of the methanol extract of Pyracantha coccinea fruit. Antioxidant properties of aqueous and methanol extracts of this plant compared to standard antioxidant BHT was very weak. Also, high levels of total phenolics content in the extracts were not observed. Such research can screening the effective and ineffective plant and extracts of different species plants and pave the way for the investigation of more than in the pharmacy.
Keywords
Fruit; Cytotoxic; Hela; Pyracantha Coccinea
Download this article as:| Copy the following to cite this article: Vahabi L, Monajemi R, Hosseini S. A. The Cytotoxic Effect of Methanolic Extract of Pyracantha coccinea M. Roemer Fruit on Hela cell line, Antioxidant Capacities and Total Phenol Contents of Methanolic and Aquatic Extract of this fruit. Biomed Pharmacol J 2015;8(March Spl Edition) |
| Copy the following to cite this URL: Vahabi L, Monajemi R, Hosseini S. A. The Cytotoxic Effect of Methanolic Extract of Pyracantha coccinea M. Roemer Fruit on Hela cell line, Antioxidant Capacities and Total Phenol Contents of Methanolic and Aquatic Extract of this fruit. Biomed Pharmacol J 2015;8(March Spl Edition). Available from: http://biomedpharmajournal.org/?p=2228> |
Introduction
Due to limitations in conducting clinical studies, the trend of carrying out researches on cancer treatment is considered to be more complicated compared to therapeutic investigations on other diseases. The main limitation is in killing or deactivating tumor cells in the presence of natural cells without the outbreak of cytotoxicity. Yet, the necessity of gaining and developing anticancer medication from natural resources such as plants requires the screening experiments of herbal extracts (Hanskell, 1995). Cytotoxic factors are active substances directly killing cells. For instance, they affect the different stages of synthesis and activity of nucleic acids and hinder cell division (Rezaeipoor, 1996).
Oxidation process is one of the factors interrupting cell cycle and inducing cancer. The factor is the major reason underlying the production of free radicals in food, medicines, and even living beings. The imbalance between the production of free radicals and antioxidant immunity systems can also lead to the damage of critical vital macromolecules like lipids, proteins, and nucleic acids (Poulson et al. 1998). Consequently, it leads to a hundred of diseases in human including arthrosclerosis, diabetes, arthritis, anemia, irrecoverable tissue damages, central nervous system damages, gastritis, and cancer. Today, natural antioxidants existing in foods and plants have drawn considerable attention. This is because they have fewer side-effects (Maxell, 1995).
The antioxidant and antibacterial activities of verbal extracts are the basic reasons for the wide application of plants in pharmaceutics, medicine, and natural therapies (Abiy et al., 2005). The Pyracantha coccinea (Rosaceae) plant is an evergreen plant with the Firethorn common name known. This plant is usually cultivated as an ornamental plant (Pignatti, 1982). Also fruit of this plant in traditional medicine used for diuretic, cardiac and tonic properties (Kowaleuki, Mrugasiewicz, 1971).
In the adult plants, the presence of flavonoids both in the hypogeal and in the aerial parts, although the qualitative compositions were very different (Bilia et al., 1991, 1993, 1994).
In this study, Cytotoxic effect of Methanolic Extract of Pyracantha coccinea fruit on Hela cell line using MTT method was examined. Also Antioxidant Capacities and Total Phenol Contents of Methanolic and Aquatic Extract of this fruit was measured.
Materials and Methods
To accomplish the objective of the study, the fruit of Pyracantha coccinea was collected from Isfahan and confirmed by falavarjan university herbarium. To prepare methanolic and aquatic extracts, 5g of the dried fruit powder was separately kept in 20 ml methanol and distilled water for 24h in dark shaking conditions. Then, resulting solution was filtered and poured in a plate to get dried in desirable thermal conditions.
Free radicals scavenging capacity was measured using DPPH method. Here, BHT was taken as standard. To set the percentage of DPPH free radicals scavenging capacity by the aquatic and methanolic extracts, various concentrations of both were prepared. And, the scavenging capacity was examined for each concentration in triplet. Then, test tubes were maintained in darkness and in permanent shaking conditions for 1h. And, the extent of solutions optical density at 517 nm was read using spectrophotometer.
Based on the optical densities gained, DPPH free radicals scavenging capacity was calculated using the following formula:
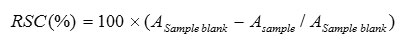
Where A Sample blank and A Sample blank stand for the level of optical density respectively in negative control and samples (Mirzaei and et al, 1390).
The antioxidant and free radicals scavenging activities of the extracts and standard were mentioned as IC50 values. The value indicated a density of the compound scavenged 50% DPPH free radicals.
To determine the total phenol content of aquatic and methanolic extracts, gallic acid standard curve and Folin-Ciocalteu reagent were applied.
Optical density of solutions was read at 765 nm after an hour of incubation. Total phenol content was calculated based on gallic acid standard curve.
To investigate the cytotoxic effect of Pyracantha coccinea fruit of methanolic extract, Hela cell line (cervical cancer) was prepared from Pasteur Institute of Iran.
To assess the cytotoxic effect of Pyracantha coccinea fruit methanolic extract, the line was derived from cervical cancer cells Hela in flask from Iran Pastor Institute.Then cytotoxic effect of Pyracantha coccinea fruit of different concentrations of methanol extract on Hela cell line at 72 hours with MTT method was examined.
Depending on their growth rate, cells covered the surface of flask a few days later in a culture medium containing 500 ml RPMI and 50 ml FBS as well as 10% antibiotics. After, taking nutrients, they turned into yellow. Now, cells had to be dissociated. To remove adherent cells from a culture surface, Trypsin 0.25% was applied to transform them into cell suspension after 5 and 10 min. Then, flasks were taken out of incubator. The cell suspension was transferred to falcon and centrifuged by 1300 rpm for 5 min. the deposited cells were cleansed by PBS. And, after being added new culture medium and its vortex, they were poured in new flasks and transferred into CO2 incubator (Bonifacino et al., 2004). When the number of cells increased and they were in incremental grow phase, cells were counted by haemocytometer slide. The cells were examined in term of cytotoxic effect using MTT method 72h after being transferred into 96-well plate (we administered the test on cells 24h after being transferred into 96-well plate. That is, at the time when the shock from cell passage was removed from cells).
Results
Methanolic extract of Pyracantha coccinea frui in 800 g/ml had more cytotoxic effect on Hela cell line (cervical cancer) (Fig 1).
Besides, DPPH free radicals scavenging capacity by methanolic and aquatic extracts was also compared to BHT free radicals scavenging capacity (as the standard) in mg/ml (Fig 2). IC50 value for BHT was calculated as 0.406 mg/ml. The value for methanolic and aquatic extracts was set as 4.61 mg/ml and 4.912 mg/ml respectively. Based on results, DPPH free radicals scavenging capacity by methanolic and aquatic extracts was lower than the standard capacity and consequently the IC50 value calculated for the extract was higher.
In addition, to determine the total phenol content using Folin-Ciocalteu reagent and gallic acid standard Diagram. Total phenol content of Pyracantha coccinea fruit methanolic and aquatic extracts were calculated as 71.56 ml and 39.78 ml of dry extract on per gram of gallic acid (Fig 4).
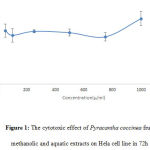 |
Figure 1: The cytotoxic effect of Pyracantha coccinea fruit methanolic and aquatic extracts on Hela cell line in 72h |
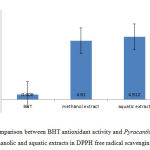 |
Figure 2: A comparison between BHT antioxidant activity and Pyracantha coccinea fruit methanolic and aquatic extracts in DPPH free radical scavenging test |
 |
Figure 3: Gallic acid standard |
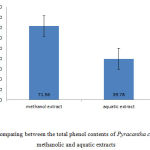 |
Figure 4: Comparing between the total phenol contents of Pyracantha coccinea fruit methanolic and aquatic extracts |
Discussion
Free radicals scavenging capacity has reverse relationship with IC50 value. Hence, as the DPPH free radical scavenging percent increases, IC50 value decreases. The percent of free radicals scavenge by methanolic and aquatic extracts as well as IC50 value for the extracts indicate the release of further antioxidant compounds in alcoholic solvent compared to aquatic solution (Fig 2).
Since methanol has lower polarity (compared to water), methanol solvent leads to the formation of compounds with more desirable polarity. It also resolves further amounts of the plant antioxidants with lower polarity. And, finally, the methanolic extract of the plant fruit contains higher antioxidant contents as compared to aquatic extract.
In this study, to determine the total phenol content of Pyracantha coccinea fruit, it was found out that methanolic and aquatic extracts do not contain high phenol contents. But, the content was significantly higher in methanolic extract compared to the aquatic extract. The amount was calculated based on the milligram of total phenol on the gram of the dried extract (Fig 4). The value indicates that methanolic solvent performs better than aquatic solvent in extracting phenols from Pyracantha coccinea fruit. Phenolic compounds have high antioxidant properties. They are effective in removing and preventing from the formation of free radicals. That is, as well as omitting them, they lead to the deposition of oxidant elements like iron (Williams and et al., 2004).
Aquatic solution is the most polar solvent. It is the extraction factor of polar phenolic compounds including tannins, caffeic acid, ferulic acid, chlorogenic acid, rosmarinic acid, and p-Coumaric acid. As a polar solvent, water can resolve some impurities such as soluble organic acids, proteins, and carbohydrates. In comparison with methanol polar solvent, it can interfere in determining and setting the amounts of phenol compounds. Perhaps, it is for the same reason that the amount of antioxidant property and total phenol contents measured in the aquatic extract are allocated less amounts (Chirinos and et al., 2007).
Results of this study showed cytotoxic methanol extract of Pyracantha coccinea fruit had no considerable cytotoxic effect.
Regarding the fact that usually the cytotoxic effects of anticancer compounds are directly related to antioxidant compounds, and regarding the lack of antioxidant property and phenol content existing in the aquatic and methanolic extracts of this fruit, the lack of cytotoxic effects in this fruit is acceptable. So that after cell culture, the methanol extract not only does not have cytotoxic effect but also does not have growth hindering effect, and cells growth continue naturally.
Such studies can lead to screening and separating active and non-active extracts from different plants genes and species and consequently pave the way for further researches concerning pharmaceutics.
References
- Abiy Y, Solomon D, Jacob O, M, Christine C.B, Matthias H, Martin G.P. 2005. Antimicrobial flavonoids from the stem bark of erythrina burtii. Department of Obstetrics and Gynecology, University of Tuebingen, Tuebingen, Germany Fitoterapia.
- Bilia, A.R., Catalano, S., De Simone, F., Morelli, I., Pizza, C., 1991. An acetylated #avanone glucoside from leaves of Pyracantha coccinea. Phytochemistry 30, 3830}3831.
- Bilia, A.R., Catalano, S., Pistelli, L., Morelli, I., 1993. Flavonoids from Pyracantha coccinea roots. Phytochemistry 33, 1449}1452.
- Bilia, A.R., Morelli, I., Marsili, A., 1994. Two glicosides from Pyracantha coccinea roots: a new lignan and a new chalcone. Tetrahedron 50, 5181}5188.
- Bonifacino J.S, Dasso M, Harford J.B, Lippincott-Schwartz J,Yamada KM. 2004. Short Protocols in Cell Biology. USA: JohnWiley& Sons, 826 P.
- Chirinos R, Rogez H, Campos D, Pedreschi R and Larondelle Y. 2007. Optimization of extraction cnditions of antioxidant phenolic compounds from mashua(Tropaeolum tuberosum Ruiz and Pavon). eparation and Purification Technology, 55: 217-225.
- Hanskell CM. Cancer Treatment. 4th ed. W. B. Saunders Company. USA. 1995, pp: 31 – 57.
- Kowaleuki, Z., Mrugasiewicz, M., 1971. Neue #avanonheteroside in Crataegus phenophyrum. Planta Medica 19, 311}313.
- Maxell S.R. 1995. Prospects for the use of antioxidants therapies. Drugs, 49: 345-61.
- Mirzaei A , Mohammadi J, Mirzaei N and Mirzaei M. 1390. The Antioxidant Capacities and Total Phenolic Contents of Some Medicinal Plants in Iran. Journal of Medical Sciences of Fasa, (3): 160-157.
- Pignatti, S., 1982. Flora d’Italia, Edagricole, Bologna, Italia, p. 610.
- Poulson H.E, Prieme H, Loft S. 1998. Role of oxidative DNA damage in cancer initiation and promotion. European Journal of Cancer Prevention, 7: 9-16.
- Rezaeipoor-Kardost R. Cytikines and therapy. Fatemieh University of Medical Sciences. Iran. 1996, pp: 61 – 95.
- Williams RJ, Spencer JPE, Rice EC. 2004. Flavonoids: Antioxidants or signaling molecules? Free Radical Biology and Medicine, 36: 838-849.







