Manuscript accepted on :March 10, 2015
Published online on: --
Plagiarism Check: Yes
Zahra Shakarami1, Mansour Zabihzadeh1, 2*, Seyyed Rabi Mahdavi3, Mohammad Reza Ay4
1 Department of Medical Physics, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. 2 Department of Radiotherapy and Radiation Oncology, Golestan Hospital, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. 3Department of Medical Physics, School of Medicine, Iran University of Medical Sciences, Tehran, Iran. 4Department of Medical Physics and Biomedical Engineering, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran.
Abstract
Applying of high-energy photon beams beside all advantages obstacled by photoneutrons that may cause extra dose to the patient that has not been considered in routine radiotherapy. The purpose of this study is calculation of neutron and gamma doses to a female undergoing a pelvic 18 MV irradiation. A simplified Linac head model as a sphere with 10 cm radius of tungsten and with the total spectrum of an isotropic neutron distribution was located inside a typical bunker. The female anthropomorphic phantom was irradiated with equal weighted four-field pelvic box (18MV). MCNPX (2.4.0) code was used to calculate of absorbed doses. The greatest effective dose, 1.04 mSv Gy-1, was calculated for the AP field while the lowest effective dose, 0.36 mSv Gy-1, was obtained for the RL field. The Percent risk of fatal second malignancy of neutron contamination following a 70 Gy x-ray treatment dose (with equal weights for each field, 17.5 Gy) is 0.152 %, including 0.056 % for the AP field, 0.033 % for the PA field, 0.031 % for the RL field and 0.032 % for the LL field. If this dose delivered only with the AP field, the risk would be 0.224 %, which is 32 % higher than that is in case of 4-field irradiation. Our present analysis shows that this simplified model can be used to estimating of photoneutron doses.
Keywords
Photoneutron dose; The fatal secondary malignancy risk; Monte Carlo simulation
Download this article as:| Copy the following to cite this article: Shakarami Z, Zabihzadeh M, Mahdavi S. R, Ay M. R. Monte Carlo Calculation of Neutron Doses to Organs of a Female Undergoing a Pelvic 18 MV Irradiation. Biomed Pharmacol J 2015;8(March Spl Edition) |
| Copy the following to cite this URL: Shakarami Z, Zabihzadeh M, Mahdavi S. R, Ay M. R. Monte Carlo Calculation of Neutron Doses to Organs of a Female Undergoing a Pelvic 18 MV Irradiation. Biomed Pharmacol J 2015;8(March Spl Edition). Available from: http://biomedpharmajournal.org/?p=2207> |
Background
In radiotherapy, for deep-seated tumors, e.g. in the pelvic region, higher photon energies such as 18 MV are widely used because of their lower integral dose and better therapeutic gain (1). The main obstacle is the production of neutrons through interactions of photon (>8 MV) with high atomic number (Z) materials of accelerator structure and the treatment room, as well as within the patient body (2, 3). Furthermore, gamma rays from neutron capture reactions are also generated in both the linac head and the patient body. The dose from these contaminant neutrons should be considered in patient extra-target calculation due to their high relative biological effectiveness values (WR ≥ 5).
As far as we know photoneutron doses, its related effective dose and the risk of developing a fatal secondary malignancy due to contaminant neutrons from female-pelvic irradiation has not investigated yet.
Considering the limitations and complications associated with neutron measurement (an accuracy better than 10% can rarely be achieved) especially in medical fields (3), the MC approach (4) has shown more flexibility and capabilities in calculating dosimetric quantities required in medical dosimetry. In the present study, using the simplified model linac’s head validated for photoneutrons (5), the mean absorbed dose in an organ, DT, the equivalent dose in an organ, HT, and the effective dose, E, from the neutron contaminant have been calculated by Monte Carlo method for a female-pelvis irradiation with 18 MV x-ray beam. Doses from capture gamma rays and neutrons were calculated, separately.
Objectives
The purpose of this study is the calculation of neutron and gamma doses to chosen organs from18 MV X-ray female pelvic irradiation and the risk of developing a fatal secondary malignancy due to contaminant neutrons.
Material and methods
In the current study, the general-purpose MCNPX (2.4.0) Monte Carlo code was used to simulate radiation transport (4). The neutron doses calculated by the f6 tally (a track-length estimator based on the use of the restricted or total stopping power of the particle). The ENDF/B and RMCCS cross section data files were used. Each simulation was performed with 109 histories as a compromise of relative errors of results and run time.
The total spectrum of an isotropic neutron distribution from the accelerator head can be obtained by considering the relative contribution of two mechanisms, evaporation contribution and direct process, as follow:
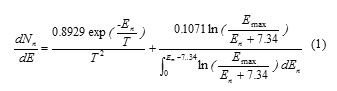
Where En is the neutron energy (in MeV), T is the nuclear temperature of the neutrons from a particular nucleus (in MeV), Emax is the maximum energy of the photons (in MeV) and the constant of 7.34 denote the average binding energy of emitted neutrons from the tungsten (in MeV) (6, 7). By considering T= 0.5 MeV and Emax =18 MeV, the photoneutron spectrum can be expressed as:
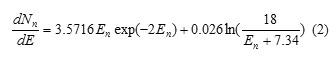
Linac head can be modeled as a 10 cm sphere of tungsten and the neutron source was considered as an isotropic point-like source, its spectrum given by Equation 2, located at the center of it (5, 7-9). A variable cone aperture was defined in the wall of tungsten sphere to provide various field size dimensions. Full bunker walls (2.26 g cm -3 concrete) and door 1.7 cm lead sandwiched between two 20 cm thickness paraffin layers) were simulated.
The anthropomorphic phantom was modeled on the basis of quadratic and planar equations according to the ORNL report (10). Specifications for the elemental compositions of lung (0.296 g cm -3), skeletal (1.40 g cm -3) and soft tissue (1.04 g cm -3) were obtained from ICRU-44 report (11). The pelvis was irradiated with four fields (10 ×10 cm2) of antero-posterior (AP, 0°), postero-anterior (PA, 180°), right lateral (RL, 270°) and left lateral (LL, 90°) in SSD (source to surface distance)=100cm. The related doses were calculated for each of these four field with delivering of 17.5 Gy (from 25 fractions of 70 MU; 1 MU= 1 cGy ) to the isocenter. Furthermore, in a separate simulation it was supposed that the total dose of 70 Gy (4´17.5 Gy) delivered by a single field (AP) in order to compare of our data with other’s data. As an aid to clinical applications, the neutron source strength (Q) value for Varian-2300 CD model (18 MV), 0.95 × 1012 neutron per Gy reported by Followill et al. (2003), was used to convert the MCNPX output from deposited energy per incident neutron to deposited energy per 1Gy x-ray dose at the isocenter (12).
Neutrons were transported without any lower energy-cutoff while gamma captured photons were transported down to the energy of 0.01 MeV. The total equivalent dose, HT, in each organ, T, across the total interval energy was estimated using the following equation:
![]()
Where En is the neutron energy of the ith neutron energy interval, DT (En) is the absorbed dose of organ T for ith neutron energy interval, is the equivalent dose of organ T from neutrons and is the equivalent dose of organ T from secondary photons (13). The appropriate radiation weighting factors, WR (En), for neutrons recommended by ICRP-60 report (1991) (13) were derived from the following equation:
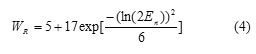
The effective dose was calculated by multiplying the calculated total equivalent doses to organs by the organ-weighting factor, WT, as recommended by ICRP-60 report (1991) (13).
These risk coefficients (from NCRP-116 report) have been used as the most commonly in calculating the risk of developing a fatal secondary malignancy (14).
Table 1: The absorbed dose per unit of x-ray treatment dose (µGy Gy-1) from neutrons and gamma capture rays in the female organs for AP, PA, RL and LL projections. For each organ, the upper number is neutrn dose and the underlined number is gamma capture rays dose. The relative uncertainties are included in parenthesis
|
Organs |
Absorbed neutron dose & gamma capture rays (µGy Gy-1) | |||
| AP PA RL LL | ||||
| Ovaries | 150.64 (0.01)
160.46 (0.01) |
185.08 (<0.01)
203.36 (<0.01) |
25.8 (0.02)
90.50 (<0.01) |
26.62 (0.02)
91.38 (<0.01) |
| Bone marrow | 12.88 (<0.01)
122.72 (<0.01) |
12.44 (<0.01)
99.2 (<0.01) |
31.22 (<0.01)
56.04 (<0.01) |
32.66 (<0.01)
57.52 (<0.01) |
| Bone surface | 13.68 (<0.01)
114.30 (<0.01) |
20.66 (<0.01)
123.84 (<0.01) |
19.32 (<0.01)
44.18 (<0.01) |
19.44 (<0.01)
49.54 (<0.01) |
| Colon | 24.42 (<0.01)
119.22 (<0.01) |
8.11 (0.01)
50.20 (<0.01) |
58.74 (0.02)
89.72 (<0.01) |
59.50 (0.02)
91.48 (<0.01) |
| Lungs | 3.56 (0.04)
28.50 (0.03) |
5.12 (0.02)
36.62 (0.02) |
3.06 (0.03)
23.78 (0.03) |
2.66 (0.03)
29.24 (0.02) |
| Stomach wall | 20.44 (<0.01)
174.72 (<0.01) |
10.26 (0.03)
128.24 (<0.01) |
11.58 (0.02)
47.94 (0.01) |
11.44 (0.02)
82.06 (0.01) |
| Bladder wall | 212.98 (0.01)
193.5 (<0.01) |
59.94 (0.02)
168.94 (0.01) |
40.38 (0.02)
92.36 (0.01) |
39.52 (0.03)
98.36 (0.01) |
| Breasts | 80.40 (<0.01)
64.34 (<0.01) |
10.41 (0.02)
141.58 (0.01) |
37.62 (0.01)
28.98 (0.01) |
37.88 (0.01)
31.96 (0.01) |
| Liver | 21.54 (<0.01)
160.66 (<0.01) |
5.06 (<0.01)
117.54 (<0.01) |
11.84 (0.02)
73.46 (0.01) |
10.02 (0.01)
60.78 (0.01) |
| Esophagus | 3.98 (0.03)
23.52 (0.02) |
1.28 (0.04)
22.46 (0.02) |
1.92 (0.04)
23.96 (0.02) |
1.76 (0.04)
23.56 (0.02) |
| Thyroid | 61.40 (0.02)
137.84 (0.03) |
80.38 (0.03)
193.84 (0.04) |
30.54 (0.04)
70.42 (0.03) |
31.44 (0.04)
70.06 (0.03) |
| Skin | 115.41 (<0.01)
60.48 (<0.01) |
113.56 (<0.01)
55.28 (<0.01) |
54.12 (<0.01)
51.00 (<0.01) |
54.80 (<0.01)
50.42 (<0.01) |
| Remainder | 45.25 (<0.01)
76.69 (<0.01) |
48.67 (<0.01)
91.90 (<0.01) |
7.87 (<0.01)
56.38 (<0.01) |
9.31 (<0.01)
70.37 (<0.01) |
Table 2: The total equivalent dose (from neutrons & gamma capture rays) per unit of x-ray treatment dose (µSv Gy-1) in the female organs for AP, PA, RL and LL projections. The total relative uncertainties are included in parenthesis. The numbers in each bracket correspond to neutron and gamma capture rays relative contributions to the total equivalent doses, respectively.
|
Organs |
Total equivalent doses from neutrons & gamma capture rays (µSv Gy-1) | |||
| AP PA RL LL | ||||
|
Ovaries |
2676.91 (0.01) [0.94, 0.06] |
3303.76 (<0.01) [0.94, 0.06] | 280.44 (0.02) [0.68, 0.32] | 287.35 (0.02) [0.68, 0.32] |
| Bone marrow | 263.66 (<0.01) [0.53, 0.47] | 255.64 (<0.01) [0.61, 0.39] | 580.58 (<0.01) [90, 10] | 606.25 (<0.01) [0.91, 0.09] |
| Bone surface | 264.78 (<0.01) [0.57, 0.43] | 422.72 (<0.01) [0.71, 0.29] | 345.29 (<0.01) [0.87, 0.13] | 352.52 (<0.01) [0.86, 0.14] |
| Colon | 510.36 (0.01) [0.77, 0.23] | 170.69 (0.01) [0.71, 0.29] | 910.59 (0.02) [0.91, 0.09] | 973.62 (0.02) [0.91, 0.09] |
| Lungs | 50.57 (0.05) [0.44, 0.56] | 93.26 (0.03) [0.61, 0.39] | 54.94 (0.04) [0.57, 0.43] | 56.32 (0.04) [0.48, 0.52] |
| Stomach wall | 352.60 (0.01) [0.50, 0.50] | 206.33 (0.02) [0.38, 0.62] | 68.57 (0.02) [0.30, 0.70] | 102.44 (0.02) [0.20, 0.80] |
| Bladder wall | 3779.59 (0.01) [0.95, 0.05] | 1088.54 (0.02) [0.84, 0.16] | 675.85 (0.02) [0.86, 0.14] | 669.42 (0.03) [0.85, 0.15] |
| Breasts | 1160.91 (0.01) [0.94, 0.06] | 214.38 (0.02) [0.34, 0.66] | 531.57 (0.01) [0.95, 0.05] | 538.29 (0.01) [0.94, 0.06] |
| Liver | 353.99 (0.01) [0.55, 0.45] | 156.12 (0.01) [0.25, 0.75] | 167.59 (0.02) [0.56, 0.44] | 140.44 (0.01) [0.57, 0.43] |
| Esophagus | 68.83 (0.04) [0.66, 0.34] | 32.06 (0.04) [0.30, 0.70] | 39.32 (0.04) [0.39, 0.61] | 37.64 (0.04) [0.37, 0.63] |
| Thyroid | 685.72 (0.04) [0.80, 0.20] | 885.68 (0.05) [0.78, 0.22] | 333.06 (0.05) [0.79, 0.21] | 340.44 (0.05) [0.79, 0.21] |
| Skin | 2083.89 (<0.01) [0.97, 0.03] | 2053.56 (<0.01) [0.97, 0.03] | 993.95 (<0.01) [0.95, 0.05] | 1005.22 (<0.01) [0.95, 0.05] |
| Remainder | 827.00 (<0.01)
[0.97, 0.03]
|
874.40 (<0.01)
[0.89, 0.11]
|
160.70 (<0.01)
[0.58, 0.42]
|
183.72 (<0.01)
[0.62, 0.38]
|
Table 3: The organ equivalent dose from photoneutrons in mSv per unit photon Gy delivered to isocenter. Our results from neutrons and gamma capture photons were reported separately. Only contaminant neutrons produced the organ equivalent doses from Howell et al and Vanhavere et al.
| Organs | organ equivalent dose from photoneutrons (m Sv per unit photon Gy delivered to isocenter) | |||
| Howell et al.a | Vanhavere et al.b | Our study | ||
| Neutron | Neutron | Neutrons Neutrons + Gamma capture photons | ||
| Bladder wall | 0.345 | 3.8 | 3.59 | 3.78 |
| Liver | 0.29 | 0.4 | 0.19 | 0.35 |
| Thyroid | 1.86 | 0.4 | 0.55 | 0.69 |
| Skin | 2.3 | 1.1 | 2.02 | 2.08 |
| Stomach | 0.13 | 0.6 | 0.18 | 0.35 |
| Colon | 0.23 | 2.3 | 0.39 | 0.51 |
| Bone marrow | 0.19 | — | 0.14 | 0.26 |
| Lungs | 0.07 | 0.2 | 0.02 | 0.05 |
| Esophagus | 0.05 | 0.1 | 0.05 | 0.07 |
| Breasts | 1.86 | — | 1.1 | 1.16 |
| Remainder | 0.29 | — | 0.68 | 0.83 |
a The results reported by Howell et al. (2006) measured inside the Randro- Alderson phantom from the delivery of conventional therapy for prostate treatment plans (18 MV-Varian linac) (16).
b The results of Vanhavere et al.(2004) measured inside the Plexi-phantom from 18 MV-Varian Clinac 2100 C-D (10 × 10 cm2 field size), converted to mSv Gy-1 (17).
Table 4: Effective dose (mSv per unit photon Gy delivered to isocenter) and percent risk of fatal secondary malignancy due to contaminant neutrons.
| Field | The effective dose (mSv Gy-1 ) | The percent risk of fatal secondary malignancy (%Gy-1 ) |
| AP | 1.05 | 0.0032 |
| PA | 0.94 | 0.0019 |
| RL | 0.36 | 0.0017 |
| LL | 0.38 | 0.0018 |
Results and discussion
The calculated neutrons have a peak of 0.5 and 0.3 MeV before and after transmitted the linac head, respectively. The average energy of photoneutrons before and after crossing the head for 10, 15, 18, 20 MeV were 0.9, 1.08, 1.25, 1.31 MeV and 0.46, 0.48, 0.51, 0.53 MeV respectively. These energy ranges of neutrons have the greatest values of radiation weighting factors (15). The average photoneutron energies after filtration through the linac head reported were from 0.5 to 0.8 MeV for 10–18 MVx-ray beams (2). The mean value of ~0.5 MeV was repoeted by Howell et al. (2006) (16). Photoneutrons with energies of 1–2 MeV and 0.2 to 2 MeV were reported by NCRP-79 report before and after the filtration of linac’s head, respectively (6). Readers is referred to our pervious paper to the details of validation about our proposed simplified model (5).
The organs inside the primary beam receive greater neutron doses (table 1). For example, greater neutron doses received by the bladder for the AP field and ovaries for the PA filed. However, the colon received greater dose than the other organs in the RL and the LL field. It may due to shielding effect of organs locating above colon in the lateral fields. The bladder wall for the AP field received the greatest neutron dose, 0.213 mGy Gy-1. The skin absorbed a considerable neutron dose in all fields. The deeper organs located within the applied field received greatest photon dose (table 1). The organs far from the treatment field (exception the thyroid) and close to the skin received lower doses from these secondary photons. For example, the bladder wall in the AP, RL and LL field and the ovaries in the PA field received greater photon doses. The greatest dose received by ovaries in the AP field, 0.203 mGy Gy-1 while the smallest gamma dose for each field delivered to the esophagus.
From table 2, the greatest total equivalent dose received by the bladder wall for the AP field (3.78 mSv Gy-1), the Ovaries for the PA field (3.30 mSv Gy-1) and the skin for the RL and LL fields (0.99 mSv Gy-1 and 1.01 mSv Gy-1). Our results for neutron equivalent dose (only for the AP field) were compared with others (16, 17) in the table 3, however essential differences between these studied makes impossible a realistic comparison. The equivalent dose of remainder organs was 0.68 mSv Gy-1 and 0.29 mSv Gy-1 from Howell et al. (2006) (16) and this study, respectivly, or 1.1 mSv Gy-1 and 1.86 mSv Gy-1 for the breasts.
The photon contribution to the total equivalent dose for some deeper organs has considerable value compared to the neutron component (table 2). For example, the contribution of the photon dose reached up to 70 % for stomach in the RL field and 75 % in the PA field. Therefore, it may be crucial to calculate the gamma capture dose (from neutron interaction) and the neutron dose separately because of different radiation weighting factors (WR = 1 for photon, WR ≥ 5 for neutron). Therefore, if the gamma capture rays not taken into account separately it may result in underestimating equivalent dose especially for deep-seated organs.
The greatest effective dose, 1.04 mSv Gy-1, was calculated for the AP field and the Ovaries have the maximum contribution (0.54 mSv Gy-1) while the lowest effective dose, 0.36 mSv Gy-1, was obtained for the RL field and the colon has the maximum contribution (0.12 mSv Gy-1). The effective dose of 1.04 mSv Gy-1 (for the AP field) is in good agreement with 1.2 and 0.96 mSv Gy-1 measured by Vanhavere et al. (2004) (17) and Howell et al. (2006) (16), respectively. The effective photoneutron dose following 70 Gy (only for the AP field) was about 0.07 Sv. Delivering this dose with equal weighting factor from the four field (with equal weights for each field, 17.5 Gy) reduces the effective dose to 0.05 Sv.
The fatal secondary malignancy risk has the minimum value for the RL field (0.0017 % Gy-1) and the maximum for the AP field (0.0032 % Gy-1), see table 4. The maximum contribution of fatalities belongs to the bladder wall in the AP field, 0.011 % Gy-1. The percent risk of fatal secondary malignancy of neutron contamination following a 70 Gy x-ray treatment dose (with equal weights for each field, 17.5 Gy) is 0.152 %, which included 0.056 % for the AP field, 0.033 % for the PA field, 0.031 % for the RL field and 0.032 % for the LL field. If this dose delivered only with the AP field the risk would be 0.224 %, which is 32 % higher than that is in form of box irradiation.
Conclusion
The calculated percent risk of fatal secondary malignancy of neutron contamination following a prescriped dose of 70 Gy x-ray during a typical female pelvic irradiation was 0.152 %. We successfully built a Monte Carlo simulation model using a simplified Linac’s head to calculate photoneutron dose parameters that has not been considered in routine radiotherapy.
Acknowledgment
This study was supported financially by research affaires of Ahvaz Jundishapur University of medical sciences, Ahvaz, Iran.
Financial Disclosure
There is no financial disclosure.
References
- Pirzkall A, Carol MP, Pickett B, Xia P, Roach M, 3rd, Verhey LJ. The effect of beam energy and number of fields on photon-based IMRT for deep-seated targets. International journal of radiation oncology, biology, physics. 2002;53(2):434-42.
- Swanson WP. Estimate of the risk in radiation therapy due to unwanted neutrons. Medical physics. 1980;7(2):141-4.
- AAPM Report 19. “Neutron measurements around high energy X-ray radiotherapy machines.” American Association of Physicists in Medicine. New York: American Institute of Physics. 1986.
- Walter LS. (Ed.). LANL (Los Alamos National Laboratory) Monte Carlo N-Particle transport code system for multiparticle and high energy applications. Version 240, LA-CP-02-408, Los Alamos National Laboratory. 2002.
- Zabihzadeh M, Ay MR, Allahverdi M, Mesbahi A, Mahdavi SR, Shahriari M. Monte Carlo Estimation of Photoneutrons Contamination from High-Energy X-Ray Medical Accelators in Treatment Room and Maze: a Simplified Model. Radiation protection dosimetry. 2009.
- NCRP Report 79. “Neutron contamination from medical linear accelerators,” National Council on Radiation Protection. Bethesda, MD. 1984.
- Tosi G, Torresin A, Agosteo S, Foglio Para A, Sangiust V, Zeni L, et al. Neutron measurements around medical electron accelerators by active and passive detection techniques. Medical physics. 1991;18(1):54-60.
- Carinou E, Kamenopoulou V, Stamatelatos IE. Evaluation of neutron dose in the maze of medical electron accelerators. Medical physics. 1999;26(12):2520-5.
- Lin JP, Liu WC, Lin CC. Investigation of photoneutron dose equivalent from high-energy photons in radiotherapy. Appl Radiat Isot. 2007;65(5):599-604.
- Cristy M, Eckerman KF. Specific Absorbed Fractions of Energy at Various Ages from Internal Photon Sources. Oak Ridge National Laboratory Report ORNL/TM-8381/VI. 1987.
- ICRU Report 44. “Tissue Substitutes in radiation dosimetry and measurement,” International Commission on Radiation Units and Measurements. Bethesda, MD. 1989.
- Followill DS, Stovall MS, Kry SF, Ibbott GS. Neutron source strength measurements for Varian, Siemens, Elekta, and General Electric linear accelerators. Journal of applied clinical medical physics / American College of Medical Physics. 2003;4(3):189-94.
- ICRP Report 60. 1990 Recommendations of the International Commission on Radiological Protection,” International Commission on Radiological Protection. Oxford, UK. 1991.
- NCRP Report 116. “Limitation of Exposure to Ionizing Radiation,” National Council on Radiation Protection and Measurements. Bethesda, MD. 1993.
- Veinot KG, Hertel NE. Effective quality factors for neutrons based on the revised ICRP/ICRU recommendations. Radiation protection dosimetry. 2005;115(1-4):536-41.
- Howell RM, Hertel NE, Wang Z, Hutchinson J, Fullerton GD. Calculation of effective dose from measurements of secondary neutron spectra and scattered photon dose from dynamic MLC IMRT for 6 MV, 15 MV, and 18 MV beam energies. Medical physics. 2006;33(2):360-8.
- Vanhavere F, Huyskens D, Struelens L. Peripheral neutron and gamma doses in radiotherapy with an 18 MV linear accelerator. Radiation protection dosimetry. 2004;110(1-4):607-12.







