Manuscript accepted on :
Published online on: 25-12-2015
Plagiarism Check: Yes
Mikhail Sergeevich Yamburov, Tatiana Petrovna Astafurova, Konstantin Vitalievich Zhuk, Svetlana Borisovna Romanova and Valentina Mikhailovna Smolina
Tomsk State University, Lenin Prospekt 36, Tomsk, 634050, Russia.
DOI : https://dx.doi.org/10.13005/bpj/526
Abstract
Many researchers analyze the effect of water deficits on microsporogenesis, although few study the influence of flooding. This study reviewed the effects of drought and flood stress on pollen quality and quantity in Clivia miniata including pollen size, viability, germination, and number of pollen grains per anther while evaluating how stress influences these features. The study provides evidence that microsporogenesis of Clivia miniata is more sensitive to flooding than to drought. The studied pollen features can be ranked based on their degree of sensitivity to flooding in the following order: number of pollen grains per anther > pollen germination > pollen fertility > pollen size. A newly modified method of counting the number of pollen grains per anther was shown to be highly sensitive and can be applied to evaluate pollen productivity using a small number of plants.
Keywords
Clivia miniata; Microsporogenesis; Pollen germination; Pollen productivity; pollen viability
Download this article as:| Copy the following to cite this article: Yamburov M. S, Astafurova T. P, Zhuk K. V, Romanova S. B, Smolina V. M. The Effects of Drought and Flood Stress on Pollen Quality and Quantity in Clivia miniata (Lindl.) Bosse (Amaryllidaceae). Biomed Pharmacol J 2014;7(2) |
| Copy the following to cite this URL: Yamburov M. S, Astafurova T. P, Zhuk K. V, Romanova S. B, Smolina V. M. The Effects of Drought and Flood Stress on Pollen Quality and Quantity in Clivia miniata (Lindl.) Bosse (Amaryllidaceae). Biomed Pharmacol J 2014;7(2). Available from: http://biomedpharmajournal.org/?p=3117 |
Introduction
The life cycle plants is affected by abiotic and biotic environmental factors that influence their growth and development. Ecological factors play a significant role in the development of plant reproductive organs. The quality and quantity of pollen plants produce are very important for both wild and cultivated plant populations. Most past researcher has proven that the process of spore formation (microsporogenesis) is very sensitive to different environmental factors [1, 2]. Some factors influence microsporogenesis more strongly than others while different plants and their local ecological populations have different levels of sensitivity to various factors. For example, xerophytes, mesophytes and hygrophytes are more sensitive to drought and flooded soil conditions than other species. Microsporogenesis is sensitive to air temperature and humidity [1, 3], water conditions [1], soil fertility [4], and defoliation by herbivores [5]. The development of anthers in trees is also sensitive to microclimatic conditions in some parts of the canopy [6]. Features of pollen quality and quantity are often used in monitoring wild plants populations [7], and much more frequently used in monitoring cultivated plants. Many research studies have analyzed the influence of water conditions on microsporogenesis but most of them have considered the influence of water deficits while few address the influence of flooding.
The present study aimed to analyze the effects of drought and flood stress on pollen quality and quantity in Clivia miniata (pollen size, viability, germination, and number of pollen grains per anther) and evaluate the sensitivity of these features to these stress factors.
Materials and methods
The research was conducted in a greenhouse of the Siberian Botanical Garden of Tomsk State University, Tomsk, Russian Federation. We used 10–15-year-old plants of Clivia miniata (Amaryllidaceae) as test objects. Plants were grown in 12-liter pots. Two months prior to anthesis we divided the plants into three groups of five: 1) a control group grown under moderate watering (once per week), 2) a group grown in drought conditions without water for two months, and 3) a group grown in flooded conditions where the pots were submerged in a water reservoir in which the water level reached 1/3 of the pots’ height, so the soil surface remained wet constantly. Plants of all three groups were grown under the same temperature and light conditions.
We gathered the research material at the beginning of anthesis and analyzed the following features: 1) pollen size; 2) pollen fertility; 3) pollen germination; 4) number of pollen grains per anther.
Pollen fertility and germination were studied using mixed samples of pollen collected from ten anthers of different flowers of every plant. We took five samples (one from every plant in each group) to calculate an average number of pollen grains to measure pollen fertility and germination. In every sample we studied more than 500 pollen grains and calculated the percentage of fertile and viable pollen grains. Thus, we studied 2500 pollen grains for every group.
A 1% acetocarmine solution was used to evaluate pollen fertility [8]. The solution stained fertile pollen grains, allowing us to differentiate them from the non-stained colorless sterile ones. Other solutions used for pollen fertility evaluation allowed the measurement of the size of 100 pollen grains (polar axis length, equatorial diameter, and ratio of polar axis length to equatorial diameter). Measurements were made using an Axio Lab.A1 light microscope (Carl Zeiss, Oberkochen, Germany) using Axio Vision software to receive, process, and analyze the images.
The viability was determined by in vitro germination of pollen grains in a medium containing agar (1%) and saccharose (1%). Microscope slides were coated with culture medium and pollen grains were sown on the slides. Microscope slides were placed in Petri dishes containing wet filter paper. Plants were germinated at 25°С using a thermostat model TS-1/80 SPU (Smolensk SKTB SPU, Russia). Pollen grains that formed a pollen tube that was at least two times longer than polar axis of a pollen grain were considered to be viable.
The number of pollen grains counted per anther was based on 30 anthers taken from each group of plants (six anthers of different flowers for five plants). A hemocytometer (Linza, Russia) was used to count the number of pollen grains per anther [9]; the method was modified for the present study. Closed anthers were collected from flowers that had started flowering; then the anthers were placed separately into plastic 2-ml microtubes. Anthers had been dried for 24 hours until they opened. Next, we added 1 ml of a 1% fuchsine water-based solution to stain the pollen. Then microtubes were shaken carefully by hand and 1 ml of glycerol was added to increase the viscosity of the solution and to distribute the pollen equally in the solution. A closed microtube was shaken for a few minutes with a Vortex V-1 plus (Biosan, Latvia) to release the pollen from the anthers. Pollen grains were counted under a microscope in each square of the grid of a hemocytometer, e.g., chamber volume is 0.0009 ml. The data was extrapolated to 2 ml of solution. Separate research was conducted to find necessary sample size (a number of samples from a microtube containing one anther). Using a hemocytometer we counted 100 samples of ten anthers in a control group of plants. This number of samples was considered to be a statistical population that showed the number of pollen grains per anther most accurately. The confidence interval was calculated separately for each statistical population of each anther. A random number generator was used to select data randomly with 100 repetitions and generate sample sizes containing 5, 10, 20, 30, 40, 50 and 60 items. We calculated the mean of each sample and compared it with the confidence interval of the statistical population. Figure 1 shows the dependence of the sample means (that were included in the confidence interval of each statistical population) on the number of samples from each microtube. This method show that the number of samples should not be less than 40 because at least 40 samples were required to allow 95% of means of all samples to fall into confidence interval of the statistical population.
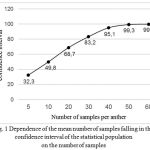 |
Figure 1: Dependence of the mean number of samples falling in the confidence interval of the statistical population on the number of samples
|
Thus, for every variation of the experiment we chose 30 anthers from different flowers. The average number of pollen grains was calculated for every anther and was based on 40 samples. The microtube was shaken with a Vortex V-1 plus before every selection to distribute the pollen evenly in the solution. When studying an anthers’ productivity, the data were often converted to that of a single flower, inflorescence or plant [2]. In our study, the groups did not have significant differences in the number of flowers per inflorescence, so we used only the initial data on the amount of pollen per one anther.
The statistical significance of the differences of studied features was determined using a Student’s t-test.
Results and Discussion
Pollen sizes of plants grown in drought and flooded conditions were a little larger than those of the control group (Table 1). Statistically significant differences were observed only in flooded conditions; in this group both polar axes and the equatorial diameter were lengthened by 6–7%. No statistically significant differences between groups were observed in the ratio of the polar axis length to equatorial diameter. Pollen in plants of both drought and flooded conditions in the experiment showed an increase in the number of sterile pollen grains and a 2–3% decrease in fertility. In addition, pollen germination had declined significantly mostly among the plants grown in flooded conditions. The 10–15% decline in pollen germination in drought and flooded conditions shows that those conditions have a significant effect on the development of male gametophytes during gamete formation. The number of pollen grains per anther was significantly smaller than the control group data in both drought (−12%) and flooding (−19%).
Table 1: Pollen quality and quantity in Clivia miniata in various hydrological conditions
| Features | Control group | Drought stress | Flood stress |
| Pollen polar axis, µm | 56.7±3.5 | 58.5±3.4 | 60.1±3.8* |
| Pollen equatorial diameter, µm | 42.1±3.0 | 43.0±2.5 | 44.9±4.1* |
| Polar axis/equatorial diameter ratio | 1.4±0.1 | 1.3±0.09 | 1.4±0.09 |
| Pollen fertility, % | 94.7±1.7 | 91.8±1.1** | 92.4±2.9* |
| Pollen germination, % | 79.6±1.9 | 72.8±4.5** | 67.2±5.5** |
| Number of pollen grains per anther × 103, p | 99.1±8.9 | 87.1±9.7** | 80.4±9.4** |
* Significant difference, p ≤ 0.05; ** p ≤ 0.01.
Thus, microsporogenesis in Clivia miniata is more sensitive to flooding than to drought. Under these conditions, the studied pollen features can vary depending on the degree of sensitivity in a decreasing order: number of pollen grains per anther > pollen germination > pollen fertility > pollen size.
The fact that Clivia miniata is a xerophyte determines its relatively low sensitivity to drought. This species has various morphological and anatomical features, such as a thick leaf cuticle, that help the species to endure long-term drought. The cuticle of Clivia miniata is > 4 µm and this reduces transpiration [10, 11, 12]. Mature pollen of some xerophytic species is also less sensitive to drought than that of mesophytes. Gullvag thoroughly described the fine structure of the pollen grains of Clivia miniata [13]. Our experiment showed that under artificial drought conditions leaves and rhizomes of Clivia miniata have water reserves and nutrients sufficient for normal development of a peduncle with flowers and the formation of pollen. Despite the species’ drought tolerance, these conditions affect pollen quality and quantity.
The high sensitivity of pollen quality and quantity of Clivia miniata to flooding is caused by the root system of xerophytes that is less tolerant to such stress conditions. Flooding leads to disturbed mineral nutrition, as well as to physiological disturbances. Factors that have an effect on plant nutrition also have an effect on pollen quality and quantity [14]. Long-term flooding can influence root system development either directly or indirectly. Flooded soil conditions directly cause root hypoxia. Even plants that are quite tolerant to flooding are affected by root hypoxia that causes a decrease in the photosynthetic rate in leaves and in the transportation of photoassimilates; these combine to result in an increase in biomass [15]. Root hypoxia influences the content of different substances in roots and leaves considerably. An experiment on Solanum lycopersicum showed that root hypoxia decreases the nitrate content in roots and leaves and increases nitrite content. It also leads to an increase in the activity of enzymes, such as nitrate and nitrite of reductases, and to a decrease in the content of water-soluble proteins [16].
Long-term flooding can also have indirect effects because a disturbance of the oxygen regime in soil negatively affects aerobic soil microorganisms. Anaerobic processes in soil acidify the soil and contribute to the accumulation of organic acids, alcohols and other compounds, some of which are toxic to plant root systems [17].
Changes caused by drought and flooding lead to changes in the balance of nutrients in plants, and therefore affect pollen quality and quantity. This occurs because pollen development occurs in anther locules that contain locular fluid and drought or flooding change the concentration of substances dissolved in that fluid [2]. During anther development plants are very sensitive to hydrological conditions. A water deficit during meiosis can increase pollen sterility and decrease pollen size. Some species did not succumb to these types of effects; other species are stress tolerant and their pollen size does not depend on water availability in reproductive tissues [1, 18].
Water deficits cause a disturbance of photosynthetic processes in assimilating tissue that leads to a decrease in the concentration of water-soluble carbohydrates and the activity of enzyme acid invertase. This decline in the activity of acid invertase restricts photoassimilation in a form that is suitable for starch biosynthesis; in addition, other processes involved in critical stages of anther and pollen development are affected [1, 18, 19].
The present study proves that flooding and drought conditions influence some features of pollen quality and quantity. Further research and a study pollen development during critical stages of microsporogenesis will be needed to obtain deeper insight into effects of these stress factors on pollen formation.
Conclusion
The present study shows that microsporogenesis of Clivia miniata is more sensitive to flooding than to drought. Under these conditions, the pollen features analyzed here can vary depending on the degree of sensitivity of various characteristics to stress in the following sequence: number of pollen grains per anther > pollen germination > pollen fertility > pollen sizes. Because the number of pollen grains produced per anther is the most sensitive feature to drought and flooding, paying greater attention to these conditions when studying the effects of these ecological stressor effect on microsporogenesis in important in both wild populations of plants or during agrocenosis of cultivated plants; for example, seed productivity depends strongly on anther productivity. The modified method of counting the number of pollen grains per anther was shown to be very sensitive and can be applied for the evaluation of pollen productivity using a small number of plants.
Acknowledgments
The Ministry of Education and Science of the Russian Federation supported this work (state registration number: 114040740044).
References
- Barnabas, B., K. Jager and A. Feher, 2008. The effect of drought and heat stress on reproductive processes in cereals. Plant, Cell and Environment, 31: 11-38.
- Khanduri, V.P., 2011. Variation in anthesis and pollen production in plants. American-Eurasian Journal of Agricultural & Environmental Sciences, 11(6): 834-839.
- Astiz, V., and L.F. Hernandez, 2014. Pollen production in sunflower (Helianthus annuus L.) is affected by air temperature and relative humidity during early reproductive growth. FYTON, 82: 297-302.
- Lau, T.C. and A.G. Stephenson, 1993. Effects of soil nitrogen on pollen production, pollen grain size, and pollen performance in Cucurbita pepo (Cucurbitaceae). American Journal of Botany, 80: 763-768.
- Lehtilä, K. and S.Y. Strauss, 1999. Effects of foliar herbivory on male and female reproductive traits of wild radish, Raphanus raphanistrum. Ecology, 80: 116-124.
- Bhattacharya, A., 2011. Does canopy height determine the pollen viability and stigma receptivity? A cross-population observation on Shorea robusta Gaertn.f. Our Nature, 9: 41-48.
- Qureshi, S.J., M.A. Khan, M. Arshad, A. Rashid and M. Ahmad, 2009. Pollen fertility (viability) status in Asteraceae species of Pakistan. Trakia Journal of Sciences, 7(1): 12-16.
- Singh, R.J., 2002. Plant Cytogenetics, second edition. CRC Press, pp: 488.
- Godini, A., 1981. Counting pollen grains of some almond cultivars by means of a haemocytometer. GREMPA, colloque. Paris: CIHEAM, Options Mediterraneennes: Serie Etudes. pp: 83-86.
- Merida, T., J. Schonherr, and H.W. Schmidt, 1981. Fine structure of plant cuticles in relation to water permeability: The fine structure of the cuticle of Clivia miniata Reg. leaves. Planta, 151: 259-267.
- Riederer, M. and L. Schreiber, 2001. Protecting against water loss: analysis of the barrier properties of plant cuticles. Journal of experimental Botany, 52(363): 2023-2032.
- Kosma, D.K. and M.F. Jenks, 2007. Eco-physiological and molecular-genetic determinants of plant cuticle function in drought and salt stress tolerance. In Advances in molecular breeding toward drought and salt tolerant crops, Eds., Jenks, M.A., P.M. Hasegawa and S.M. Jain. Springer, pp: 91-120.
- Gullvag, B., 1964. The fine structure of the pollen grain of Clivia miniata. Grana Polynologica, 5(3): 253-263.
- Cruden, R.W., 2000. Pollen grains: why so many? Plant Systematics and Evolution, 222: 143-165.
- Kogawara, S., T. Yamanoshita, M. Norisada, M. Masumori and K.Kojima, 2006. Photosynthesis and photoassimilate transport during root hypoxia in Melaleuca cajuputi, a flood-tolerant species, and in Eucalyptus camaldulensis, a moderately flood-tolerant species. Tree Physiology, 26: 1413-1423.
- Horchani, F. and S. Aschi-Smiti, 2010. Prolonged root hypoxia effects on enzymes involved in nitrogen assimilation pathway in tomato plants. Plant Signaling & Behavior, 5(12): 1583-1589.
- Sojka, R.E., D.M. Oosterhuis and H.D. Scott, 2014. Root oxygen deprivation and the reduction of leaf stomatal aperture and gas exchange. In Handbook of photosynthesis, second edition, Eds., Pessarakli, H. CRC Press, pp: 299-314.
- Saini, H.S., 1997. Effect of water stress on male gametophyte development in plants. Sexual Plant Reproduction, 10: 67-73.
- Dorian, S., S. Lalonde and H.S. Saini, 1996. Induction of male sterility in wheat by meiotic stage water deficit is preceded by a decline in invertase activity and changes in carbohydrate metabolism in anthers. Plant Physiology, 111: 137-145.







