Aniket Kumar1, Margaret Shanthi F. X1, Mahalakshmi C2, Anand Ramasamy1, Bina Isaac3 and Manoj G. Tyagi1
1Department of Pharmacology and Clinical Pharmacology, Christian Medical College, Vellore, TN, India.
2Department of Neurochemistry, Christian Medical College, Vellore, TN, India.
3Department of Anatomy, Christian Medical College, Vellore, TN, India.
Corresponding Author E-mail: tyagi257@yahoo.in
DOI : https://dx.doi.org/10.13005/bpj/539
Abstract
This study was conducted to investigate the role of endogenous tonicity responsive enhancer binding proteins (TonEBP) on normal physiology of male albino wistar rats and the possible toxic effects when this transcription factor or its transduction products are inhibited. This study was conducted on thirty six male albino rats weighing between 200-250 g, divided randomly into six groups. Rats in 1st group were used as a control, the 2nd group of rats received lithium chloride (3mEq/kg, O) every alternative day orally for 2 weeks. 3rd and 4th group of rats received a total dose of rottlerin (0.2mg/kg, I/P) and (0.5mg/kg, I/P) respectively every alternative day for 2 weeks, while 5th and 6th group rats received both lithium chloride and rottlerin (0.2mg/kg, I/P and 0.5mg/kg, I/P) respectively. The mean arterial pressure was analyzed followed by collection of blood and heart tissue for the estimation of various biochemical parameters such as amino acids, aldose reductase enzyme activity and malondialdehyde levels and kidney for histopathological observations. The study reveals that rottlerin induced inhibition of TonEBP causes deleterious effect on vascular as well as biochemical parameters in both the doses as there was increase in mean arterial pressure, significant reduction in osmoprotective amino acids like taurine, glutamate, phenylalanine and a significant increase in lipid peroxidation in heart tissue. The histopathological observations also revealed degeneration such as mild glomerular, peritubular and blood vessel congestion, presence of inflammatory cells and deposition of colloids. These finding suggests the role of TonEBP in maintenance of normal physiology during osmotic stress in experimental animals.
Keywords
TonEBP; Hypertonicity; Hyperosmolarity; Aldose reductase enzyme; Rottlerin
Download this article as:| Copy the following to cite this article: Kumar A, Shanthi F. X. M, Mahalakshmi C, Ramasamy A, Isaac B, Tyagi M. G. Rottlerin Mediated Modulation of Tonicity-Responsive Enhancer Binding Protein (TonEBP) In Wistar Rats. Biomed Pharmacol J 2014;7(2) |
| Copy the following to cite this URL: Kumar A, Shanthi F. X. M, Mahalakshmi C, Ramasamy A, Isaac B, Tyagi M. G. Rottlerin Mediated Modulation of Tonicity-Responsive Enhancer Binding Protein (TonEBP) In Wistar Rats. Biomed Pharmacol J 2014;7(2). Available from: http://biomedpharmajournal.org/?p=3246 |
Introduction
The property of water which allows it to diffuse freely across most of the membranes helps animal cells preserve balanced osmolarity for prevention of dehydration which maintains cells sustainability. In response to a hypertonic stress most of the cells are equipped with an automated process in which acute compensatory changes in cell volume leads to an intracellular accumulation of organic osmolytes such as sorbitol, myo-inositol, betaine and taurine, which restores cell volume by increasing the intracellular osmolality, and provide a protection mechanism against hyperosmotic stress1–3 which otherwise causes significant damage to proteins4 and DNA.5The accumulation of organic osmolytes is mediated by the transcription factor tonicity-responsive enhancer (TonE) binding protein (TonEBP).6 TonEBP transcribes several genes involved mainly in the accumulation of compatible intracellular organic osmolytes that compensate the extracellular hypertonic gradient. Some of these genes include Aldose reductase (AR), sodium/chloride/betaine cotransporter (BGT1), sodium/myo-inositol cotransporter (SMIT) and neuropathy target esterase NTE.7–9Expression of TonEBP has been seen during early development and in adulthood. It is expressedwidely in brain, heart, liver, and many other organs.10Gene knockout studies of TonEBP in mice demonstrate progressive disruption of kidney morphology and function after birth. The inability of cells of the kidney medulla to activate the production of organic osmolytes genes which depends on expression of TonEBP seems to be the reason for this severe kidney dysfunction in mutant mice.11Sodium excretion plays an important role in lithium treated patients which results in a condition known as acquired nephrogenic diabetes insipidus and associated polyuria. Natriuresis induced by lithium lasts for few days and is followed by retention of sodium which continues for anextended time which returns to balanced sodium levels afterwards.12,13 Retention of sodium leads to a hyperosmotic stress to cells for which the long-term adaptation is achieved by cellular accumulation of organic osmolytes.1 In our experiments, we used lithium for the development of hypertonicity and over expression of TonEBP and related transduction products and enzyme.NaCl–induced osmotic stress is the stimulusto oxidative stress14,15and high levels of it have been shown to increase reactive oxygen species (ROS) production in the kidney.16Role of TonEBP in maintenance of arterial pressure in rats have been demonstrated previously and showed that during hypertonicity the inhibition of TonEBPwith the help of doxorubicin, a drug which activates proteasome mediated proteolysis of transcriptional property of TonEBP,affected the cardiovascular function.17Limitations with cell culture studies are because of the one factor that does make a major difference and that is the time course of the osmotic change. The increase in osmotic changes is gradual in case of in vivo, not an abrupt single step as in most tissue culture experiments which increase stress on cells more by rapidly shrinking them.18While an increasing number of molecules are being proposed as playing a role in the regulation of TonEBP activity, evidences and models about how it is regulated are still deficient.19In this study, we created an in vivo model of hyperosmolarity using lithium and this was confirmed by the elevated activity of aldose reductase (AR) enzyme activity and other parameters.
We also studied the modulatory changes occuring at various physiological processes, with the help of an experimental inhibitor of TonEBP i.e. Rottlerin a compound from Mallotusphilippinensiswhich reduces TonEBP expression through a PKCδ-independent mechanism,20 which were not possible to evaluate in cultured cells. Our findings demonstrate a crucial role of TonEBP as a tonicity responsive transcription factor required for renal as well as cardiovascular homeostasis and functions at various levels.
Materials and Methods
Animals
Healthy, male albino Wistar rats each weighing 250-300 g were used for this study. The rats were housed in polypropylene cages and maintained under standard conditions (12 h light and dark cycles, at 25±3ºC and 35-60% humidity). Standard pelletized feed and tap water were provided ad libitum. The study was approved by the Institutional Animal Ethical Committee of
Christian Medical College, Vellore, India, registered under CPCSEA, India (Registration No. 88/1999/CPCSEA). All procedures were conducted in accordance with the “Guide for the Care and Use of Laboratory Animals”.
Drugs
Normal saline (0.9%), heparin, ketamine hydrochloride, diazepam, distilled water were obtained from the Hospital Pharmacy of the Christian Medical College and Hospital, Vellore, India. Other drugs like lithium chloride, potassium chloride, tricholoroacetic acid, thiobarbituric acid, β-NADPH, glyceraldehyde, lithium Sulphate, mercaptoethanol, chemicals and reagents of analytical grade were either obtained from Sigma Aldrich India or Fisher Scientific India. Rottlerin was obtained from Santa Cruz Biotechnology India.
Study Design
Animals were randomly assigned in 6 groups with each group containing 6 animals. Group 1 animals received normal saline and served as control group. Lithium chloride was given to develop hypertonicity in the dose of 3meq lithium/kg every alternative day orally for 2 weeks and served as positive control. Inhibitor of TonEBP, Rottlerin alone was given to group 3 and 4 at a dose of 0.2mg/kg and 0.5mg/kg respectively,which was neither cardiotoxic nor nephrotoxic(according to the unpublished pilot study done),in seven equally divided doses for the duration of two weeks on every alternative day. Last two groups i.e. group 5 and 6 received lithium along with rottlerin in the same above concentrations and fashion respectively. Experiments were continued for 2 weeks and on day 15th mean arterial pressure analysis was done following which the blood was collected for estimation of other biochemical parameters and finally the kidneys were removed for histopathological analysis.
Mean Arterial Pressure Analysis
Rats were anesthetized with ketamine hydrochloride (75–100 mg/kg) given via intraperitoneal route. The carotid artery was cannulated and the animals were heparinized with 0.25 ml of 1000 IU/ml of Heparin21 following which the mean arterial pressure was monitored with the help of a pressure transducer attached to data acquisition system.17After completion of this experiment the blood from rats was collected for analysis of biochemical parameters.
Biochemical Analysis
Aldose Reductase EnzymeActivity(ALR)
In tubes containing the anticoagulant blood was drawn from animals and then was subjected to centrifugation for separation of RBCs. It was further washed with saline thrice and stored for future analysis. By adding 50mM sodium phosphate buffer at pH 7.4 and 150 mM NaCl, a 10% erythrocyte suspension was made and was lysed by repeated freezing and thawing. Insoluble debris was then removed by centrifugation. ALR activity was measured spectrophotometrically using a properly diluted hemolysate22utilizinga T60 LABINDIA UV-VIS spectrophotometer.
Determination of Plasma Amino Acids
Amino acids like taurine, glutamate, phenylalanine and 15 other essential and non-essential amino acids were analyzed by HPLC method, Shimadzu LC-20AD as per the technique of Ishida et al.23
Lipid Peroxidation Determination (LPO)
The thiobarbituric acid reactive substance (TBARS) levels as guide of malondialdehyde (MDA) production were measured by the method described by Nair et al.24 in the heart tissue. Proper tissue aliquots were prepared. End product of lipid peroxidation, MDA reacts with TBA-TCA complex to form a colored compound at boiling temperature displaying absorption at 532 nm. The content of Malondialdehyde (MDA) (nmol/g wet tissue) was then calculated with an extinction coefficient ε532= 1.53X105M-1cm-1 with the help of spectrophotometer (UV-VIS T60 LABINDIA).
Analysis of Kidney Histopathology
Kidneys were sectioned longitudinally in two halves and were kept in 10% neutral formalin solution.25Both kidneys were processed and embedded in paraffin wax. With the help of microtome serial sections of 5 μm thickness were made.After which the sections were stained with hematoxylin and eosin stain and were examined under a Leitz DMRHC research microscope. A histomorphological evaluation of all the kidney sections was done in a blinded fashion by an anatomist who was unaware of the treatment groups.
Statistical Analysis
The data obtained was analyzed using one-way ANOVA followed by Tukey HSD for multiple comparison tests. P < 0.05 was considered significant. SPSS statistics (version 17.0) was used for statistical analysis.
Results
The results aredescribed in Table 1 and Figure 1, 2 and 3.
Table 1: Effect of Rottlerin on Mean Arterial Pressure and biochemical parameters in lithium treated rats.
| ALR enzyme activity (Units/min/ml) | Mean Arterial Pressure (mmHg) | Malondialdehyde (MDA)
(nmol/g wet tissue) |
|
| Control | 0.42 ± 0.01 | 95.96 ± 3.45 | 114.9 ± 1.10 |
| Lithium (Li) | 1.49 ± 0.13*** | 92.49 ± 3.10 | 117.05 ± 1.68 |
| Rot 0.2mg/kg | 0.43 ± 0.03 | 94.52 ± 1.95 | 113.03 ± 2.03 |
| Rot 0.5mg/kg | 0.44 ± 0.02 | 97.71 ± 2.83 | 115.51 ± 2.73 |
| Li + Rot 0.2mg/kg | 1.06 ± 0.05# | 103.40 ± 1.88 | 125.57 ± 3.78 |
| Li + Rot 0.5mg/kg
|
0.78 ± 0.05### | 117.02 ± 3.76### | 127.91 ± 2.11# |
Values are expressed as the mean ± SEM (n=6). One-way ANOVA with post- hoc analysis.
Rot – Rottlerin, ALR – Aldose reductase. Significant differences are indicated by *** p < 0.001, * p < 0.05 vs. control, ###p < 0.001, #p < 0.05 vs. pre-treatment lithium (positive control)
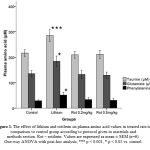 |
Figure 1: The effect of lithium and rottlerin on plasma amino acid values in treated rats in comparison to control group according to protocol given in materials and methods section. Rot – rottlerin. Values are expressed as mean ± SEM (n=6). One-way ANOVA with post-hoc analysis, *** p < 0.001, * p < 0.05 vs. control. |
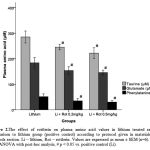 |
Figure 2: The effect of rottlerin on plasma amino acid values in lithium treated rats in comparison to lithium group (positive control) according to protocol given in materials and methods section. Li – lithium, Rot – rottlerin. Values are expressed as mean ± SEM (n=6). One-way ANOVA with post-hoc analysis, # p < 0.05 vs. positive control (Li).
|
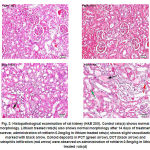 |
Figure 3: Histopathological examination of rat kidney (H&E 20X). Control rats(a) shows normal morphology. Lithium treated rats(b) also shows normal morphology after 14 days of treatment however, administration of rottlerin 0.2mg/kg in lithium treated rats(c) shows slight vacuolization marked with black arrow. Colloid deposits in PCT (green arrow), DCT (black arrow) and neutrophils infiltration (red arrow) were observed on administration of rottlerin 0.5mg/kg in lithium treated rats(d).
|
Biochemical Analysis
Aldose reductase enzyme activity in R.B.Cs was increased significantly (p < 0.001) onlyin group 2 (lithium treatment) compared to group 1 (control group). Whereas, group 3 & 4 (rottlerin 0.2mg/kg and 0.5mg/kg) respectively, did not show any change. When lithium treated animals were given rottlerin 0.2mg/kg and 0.5mg/kg (group 5 & 6 respectively), there was a reduction in enzyme activity which was significant (p < 0.05 and p < 0.001 respectively).
Analysis of plasma amino acid values of three amino acids were done and we found that there was a significant increase (p < 0.001, p <0.05 and p < 0.05) in the levels in group 2 for taurine, glutamine and phenylalanine respectively when compared to group 1. Other groups did not show any changes in its levels. Lithium treated animals when injected with rottlerin (group 5 & 6) displayed a significant reduction (p < 0.05) in all the three amino acids levels when compared to positive control (group 2).
On analysis of lipid peroxidation, it was found that the level of MDA was not different in any group except in group 6, where it showed a significant increase (p < 0.05). Similarly the level was found to be increased in group 5 as well but was not statistically significant.
Mean Arterial Pressure
Mean arterial pressure analysis of rats showed that group 6 had a significant increase (p < 0.001) when compared to group 2. Group 5 also showed an increase in the mean arterial pressure but was not significant, whereas no other group showed any alteration in the pressure analysis.
Histopathological Studies
On observations by light microscopy as shown in Figure 3, it was found that the animals on lithium treatment when given rottlerin 0.5mg/kg (group 6) showed mild morphological changes in kidney such as mild glomerular, peritubular and blood vessel congestion, presence of inflammatory cells and deposition of colloids. Only one animal from group 5 showed few alterations in kidney morphology otherwise it was normal for all the other animals in different groups.
Discussion
Hypertonicity is traumatic to the cells of all organisms.17 Cells survive by increasing the transcription of genes in a hypertonic environment whose products catalyze cellular accumulation of compatible osmolytes. Tonicity responsive enhancer binding protein (TonEBP) stimulates genes which codes for transporters and enzymes that catalyzes cellular accumulation of organic osmolytes such as the sorbitol via aldose reductase stimulation, sodium/myo-inositol cotransporter (SMIT), the sodium/chloride/betaine cotransporter (BGT1) and neuropathy target esterase (NTE) which is responsible for glycerophosphocholine production.26Hypertonicity in animals can be achieved by treating animals with lithium orally, which leads to a condition known as diabetes insipidus with marked hypertonicity.In this study, we established the model for hypertonicity in albino wistar rats by inducing polyuria with the help of lithium chloride where it says that afternatriuresis there is a phase of sodium retention which extends for few days and causes hypertonicity.12,13During the lithium treatment we observed polyuria where the amount of urine production was increased to the maximum of 4 times the normal output (data not shown). During this state of hypertonicity, it has been suggested that there is a TonEBP transcriptional factor driven accumulation of organic osmolytes such as sorbitol, myo-inositol, betaine, taurine and many other amino acids which protects the cells from osmotic stress.1–3,27It is also known that in response to hypertonicity, sorbitol is produced from glucose by the enzyme aldose reductase (AR) and there is also an upregulation of this enzyme.28,29Role of taurine in various physiological functions, such as modulation of ion movement, regulation of intracellular osmolality, conjugation with bile acids, detoxification and membrane stabilization is evident.30,31Taurine plays an important role in regulation of ion flow and influences cardiac contractility and membrane excitability.31 Not only taurine27 but it has been shown that there are various other amino acids which are synthesized and get accumulated in response to hypertonic stress e.g. arginine32, glycine33, glutamine34,35 and phenylalanine.36 The results from this study have confirmed that there is an upregulation of AR enzyme activity and also the accumulation of amino acids like taurine, glutamine and phenylalanine during hypertonicity induced by lithium for counteracting the harmful effects of osmotic stress. On the other hand, if the production of these protective osmolytes are inhibited, it could lead to alterations in normal physiological processes which could be dangerous in nature, such as renal atrophy seen in animals where the gene for TonEBP was knocked out.11 According to this study, animals in which the expression of TonEBP was inhibited with rottlerin during hypertonicity, there was reduction in the levels of AR enzyme activity which could produce sorbitol which is anosmoprotectant as well as the amino acids. In hypertonic animals, treatment with rottlerin 0.2mg/kg and 0.5mg/kg not only attenuated the AR enzyme and amino acids, but also it produced an elevation in mean arterial pressure in-vivo. This elevation could propose the possible association of TonEBP in cardiotoxicity with reduction in taurine and possibly other amino acids which could provide cardio protection. It has already been shown that chronic treatment with doxorubicin which also attenuates TonEBP and its transduction products in hypertonic animals causes elevated blood pressure.17
Cardiac cells do not experience transmembrane osmotic gradients under normal physiological conditions which could lead to swelling or shrinking of cells. Isosmotic changes occur in cardiac cell volume in few pathological conditions like diabetic coma and septic shock.37 Cardiomyocytes have a restricted proliferative potential therefore cell death leads to heart failure.38It becomes very crucial for cardiomyocytes to regulate their volume for survival which suggests the osmoregulatory role of TonEBP in heart. In this context, it is already known that taurine or TauT system protects cardiac tissues against doxorubicin induced cardiotoxicity, hypoxia-induced apoptosis, and heart failure by providing an osmotic gradient which counteracts the stress on cardiomyocytes.39,40 In the current study we found that the inhibition of AR activity and other osmoprotective amino acids during hypertonicity in group 5 and 6 lead to an increase in oxidative stress on heart and because of that there was an increase in malondialdehyde levels, a marker of lipid peroxidation, in heart cells. During the state of hypertonicity when the osmoprotective elements were not inhibited, animals showed normal physiology, which proves the crucial role of TonEBP in the heart as well.
Role of TonEBP in the development and maintenance of kidney morphology has already been published and supports that the inhibition of transcriptional response to hypertonic stress could lead to renal atrophy.11 In the present study, the biochemical modificationscorrelates with the histopathological alterations which can be seen in hypertonic animals’ where inhibition of AR and osmoprotective osmolytes produced morphological changes (group 5 and mainly in group 6) and displayedmild glomerular, peritubular and blood vessel congestion, presence of inflammatory cells and deposition of colloids.The evidence so far indicates that TonEBP/NFAT5 is expressed in virtually all tissues suggests however that its function is not limited to the renal medulla. Current data suggests the critical roles of TonEBP/NFAT5-dependent gene regulation in various tissues. Several genes not involved in osmo-adaptation are known to be the target of TonEBP. These include genes involved in embryogenesis and development, inflammation, proliferation, hepatic detoxification, extracellular matrix production, and others.41 There is increasing optimism about the prospects of the transcription factor TonEBP (NFAT5) in inflammatory diseases including atherosclerosis, rheumatoid arthritis, systemic lupus erythematosus and chronic kidney disease. There are prospects of treating these disorders with modification of TonEBP activity.
Conclusion
The current study, demonstrates the role and importance of TonEBP during hypertonicity, in an in-vivorat model. At present most of the published works ascertaining the role of TonEBP is either on cultured cells or is limited to the effects on kidneys per se.With the help of this animal model of hypertonicity, we could elucidate the effects of TonEBP and its transduction elements on various physiological processes i.e. on mean arterial pressure, production of various osmoprotective amino acids, oxidative stress encountered by cardiomyocytes during hypertonicity and in maintenance of kidney morphology in-vivo.
Acknowledgments
We are thankful to the Institutional Review Board, Christian Medical College, Vellore for providing the financial support for this study. The author is also thankful to Mr. Soosai Manickam A., Department of Physiology, Christian Medical College, Vellore for his help during the course of this study.
References
- Burg, M. B., Kwon, E. D. & Kültz, D. Regulation of gene expression by hypertonicity. Annu. Rev. Physiol.59, 437–455 (1997).
- Häussinger, D. The role of cellular hydration in the regulation of cell function. Biochem. J.313 ( Pt 3), 697–710 (1996).
- Kwon, H. M. & Handler, J. S. Cell volume regulated transporters of compatible osmolytes. Curr. Opin. Cell Biol.7, 465–471 (1995).
- Bolen, D. W. Protein stabilization by naturally occurring osmolytes. Methods Mol. Biol. Clifton NJ168, 17–36 (2001).
- Kültz, D. & Chakravarty, D. Hyperosmolality in the form of elevated NaCl but not urea causes DNA damage in murine kidney cells. Proc. Natl. Acad. Sci. U. S. A.98, 1999–2004 (2001).
- Ferraris, J. D. et al. Activity of the TonEBP/OREBP transactivation domain varies directly with extracellular NaCl concentration. Proc. Natl. Acad. Sci. U. S. A.99, 739–744 (2002).
- Miyakawa, H. et al. Cis- and trans-acting factors regulating transcription of the BGT1 gene in response to hypertonicity. Am. J. Physiol.274, F753–761 (1998).
- Rim, J. S. et al. Transcription of the sodium/myo-inositol cotransporter gene is regulated by multiple tonicity-responsive enhancers spread over 50 kilobase pairs in the 5’-flanking region. J. Biol. Chem.273, 20615–20621 (1998).
- Kempson, S. A., Zhou, Y. & Danbolt, N. C. The betaine/GABA transporter and betaine: roles in brain, kidney, and liver. Integr. Physiol.5, 159 (2014).
- Maouyo, D. et al. Mouse TonEBP-NFAT5: expression in early development and alternative splicing. Am. J. Physiol. Renal Physiol.282, F802–809 (2002).
- López-Rodríguez, C. et al. Loss of NFAT5 results in renal atrophy and lack of tonicity-responsive gene expression. Proc. Natl. Acad. Sci. U. S. A.101, 2392–2397 (2004).
- Lazarus, J. H. & Collard, K. J. Endocrine and Metabolic Effects of Lithium. (Springer Science & Business Media, 1986).
- Bateman, A. M., Larner, A. J., McCartney, S. A. & Rifkin, I. R. Rhabdomyolysis associated with lithium-induced hyperosmolal state. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. – Eur. Ren. Assoc.6, 203–205 (1991).
- Qin, S., Ding, J., Takano, T. & Yamamura, H. Involvement of receptor aggregation and reactive oxygen species in osmotic stress-induced Syk activation in B cells. Biochem. Biophys. Res. Commun.262, 231–236 (1999).
- Zhang, Z., Yang, X. Y. & Cohen, D. M. Urea-associated oxidative stress and Gadd153/CHOP induction. Am. J. Physiol.276, F786–793 (1999).
- Yang, T. et al. Hypertonic induction of COX-2 in collecting duct cells by reactive oxygen species of mitochondrial origin. J. Biol. Chem.280, 34966–34973 (2005).
- Kumar, A., Winston, A. B., Mahalakshmi, C., Amirtham, S. M. & Tyagi, M. G. In vivo study of the role of Tonicity-responsive Enhancer Binding Protein (TonEBP) and its modulation, on biochemical parameters in rats. Biomed. Res.25, 289–296 (2014).
- Lohr, J. W. & Grantham, J. J. Isovolumetric regulation of isolated S2 proximal tubules in anisotonic media. J. Clin. Invest.78, 1165–1172 (1986).
- Cheung, C. Y. & Ko, B. C. NFAT5 in cellular adaptation to hypertonic stress – regulations and functional significance. J. Mol. Signal.8, 5 (2013).
- Zhao, H., Tian, W. & Cohen, D. M. Rottlerin inhibits tonicity-dependent expression and action of TonEBP in a PKCdelta-independent fashion. Am. J. Physiol. Renal Physiol.282, F710–717 (2002).
- Parasuraman, S. & Raveendran, R. Measurement of invasive blood pressure in rats. J. Pharmacol. Pharmacother.3, 172–177 (2012).
- Reddy, G. B. et al. Erythrocyte aldose reductase activity and sorbitol levels in diabetic retinopathy. Mol. Vis.14, 593–601 (2008).
- Ishida, Y., Fujita, T. & Asai, K. New detection and separation method for amino acids by high-performance liquid chromatography. J. Chromatogr.204, 143–148 (1981).
- Nair, V. & Turner, G. A. The thiobarbituric acid test for lipid peroxidation: Structure of the adduct with malondialdehyde. Lipids19, 804–805 (1984).
- Ogeturk, M. et al. Caffeic acid phenethyl ester protects kidneys against carbon tetrachloride toxicity in rats. J. Ethnopharmacol.97, 273–280 (2005).
- Miyakawa, H., Woo, S. K., Dahl, S. C., Handler, J. S. & Kwon, H. M. Tonicity-responsive enhancer binding protein, a rel-like protein that stimulates transcription in response to hypertonicity. Proc. Natl. Acad. Sci. U. S. A.96, 2538–2542 (1999).
- Law, R. O. Amino acids as volume-regulatory osmolytes in mammalian cells. Comp. Biochem. Physiol. A99, 263–277 (1991).
- Burg, M. B., Kwon, E. D. & Kültz, D. Osmotic regulation of gene expression. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol.10, 1598–1606 (1996).
- Woo, S. K. & Kwon, H. M. Adaptation of kidney medulla to hypertonicity: role of the transcription factor TonEBP. Int. Rev. Cytol.215, 189–202 (2002).
- Azuma, J., Sawamura, A. & Awata, N. Usefulness of taurine in chronic congestive heart failure and its prospective application. Jpn. Circ. J.56, 95–99 (1992).
- Schaffer, S., Azuma, J., Takahashi, K. & Mozaffari, M. Why is taurine cytoprotective? Adv. Exp. Med. Biol.526, 307–321 (2003).
- Aoki, E. & Takeuchi, I. K. Immunohistochemical localization of arginine and citrulline in rat renal tissue. J. Histochem. Cytochem. Off. J. Histochem. Soc.45, 875–881 (1997).
- Dawson, K. M., Collins, J. L. & Baltz, J. M. Osmolarity-dependent glycine accumulation indicates a role for glycine as an organic osmolyte in early preimplantation mouse embryos. Biol. Reprod.59, 225–232 (1998).
- Loyher, M. L., Mutin, M., Woo, S. K., Kwon, H. M. & Tappaz, M. L. Transcription factor tonicity-responsive enhancer-binding protein (TonEBP) which transactivates osmoprotective genes is expressed and upregulated following acute systemic hypertonicity in neurons in brain. Neuroscience124, 89–104 (2004).
- Jiang, S. et al. Glutamate release through connexin 43 by cultured astrocytes in a stimulated hypertonicity model. Brain Res.1392, 8–15 (2011).
- Brocker, C., Thompson, D. C. & Vasiliou, V. The role of hyperosmotic stress in inflammation and disease. Biomol. Concepts3, 345–364 (2012).
- Wright, A. R. & Rees, S. A. Cardiac cell volume: crystal clear or murky waters? A comparison with other cell types. Pharmacol. Ther.80, 89–121 (1998).
- Bing, O. H. Hypothesis: apoptosis may be a mechanism for the transition to heart failure with chronic pressure overload. J. Mol. Cell. Cardiol.26, 943–948 (1994).
- Hamaguchi, T. et al. Protective effect of taurine against doxorubicin-induced cardiotoxicity in perfused chick hearts. Pharmacol. Res. Off. J. Ital. Pharmacol. Soc.21, 729–734 (1989).
- Takatani, T. et al. Taurine prevents the ischemia-induced apoptosis in cultured neonatal rat cardiomyocytes through Akt/caspase-9 pathway. Biochem. Biophys. Res. Commun.316, 484–489 (2004).
- Neuhofer, W. Role of NFAT5 in inflammatory disorders associated with osmotic stress. Curr. Genomics11, 584–590 (2010).








