Manuscript accepted on :
Published online on: 24-12-2015
Plagiarism Check: Yes
Najafian Mahmood1, Mokaber Haleh2, Pourahmadi Mohammad3, Farzam Mohsen4 and Kargar Jahromi Hossein3
1Department of Biology, Jahrom Branch, Islamic Azad University, Jahrom, Iran.
2Department of Developmental Biology, Science and Research Branch, Islamic Azad University, Fars, Iran.
3Department of Anatomy, Jahrom University of Medical Science, Jahrom, Iran.
4Department of Veterinry, Kazeroon Branch, Islamic Azad University, Kazeroon, Iran.
DOI : https://dx.doi.org/10.13005/bpj/496
Abstract
The most vulnerable tissue against damages and side effects of medications is the liver tissue. Among the medications, most frequently used ones are antibiotics with among which toxicity with aminoglycosides, especially gentamicin has special importance. Studies have shown that gentamicin exerts its hepatotexicity poperty through creating free radicals. Cinnamon has antioxidant properties. In This research the antioxidant properties of cinnamon in reducing hepatotexicity poperty caused by gentamicin is studied. In this study, effect of gentamicin as 100mg/kg-BW (G100), cinnamon extract as 200mg/kg-BW (C200) and gentamicin with the dosages of 50, 100 and 200mg/kg-BW of cinnamon extract (GC50, GC100 and GC200) on rats was studied. The results of research show severe destroy of tissue in the liver in group G100. Activity of the pyruvate transaminase enzyme, exhalostat transaminase, and fosfatase alcaline in group G were significantly increased (P <0.05) compared to control group. In group GC50, GC100 and GC200, roughly proportional to the dose of cinnamon extract, enzyme activity reduced compared to group G (P <0.05). The tissue results also indicate tissue damage caused by gentamicin and healing by antioxidant properties of Cinnamon in group GC200. It may be concluded that cinnamon extract with its antioxidantproperties reduces liver damage caused by gentamicin.
Keywords
Hepatoxicity; Gentamicin; Cinnamon; Rats
Download this article as:| Copy the following to cite this article: Mahmood N, Haleh M, Mohammad P, Mohsen F, Hossein K. J. Pathological Changes of Gentamicin in Liver Tissue and Antioxidant Property of Cinnamon Extract on Wistar Rats. Biomed Pharmacol J 2014;7(1) |
| Copy the following to cite this URL: Mahmood N, Haleh M, Mohammad P, Mohsen F, Hossein K. J. Pathological Changes of Gentamicin in Liver Tissue and Antioxidant Property of Cinnamon Extract on Wistar Rats. Biomed Pharmacol J 2014;7(1). Available from: http://biomedpharmajournal.org/?p=2985 |
Introduction
One of the most widely used class of drugs are antibiotics. These drugs prevent many problems caused by infections. However, antibiotics have side effects and can damage various body organs including liver, kidney, brain, blood, skin, eyes, mouth, etc. (1). Aminoglycosare antibiotics, especially gentamicin are widely used to treat severe infections of Gram-negative bacteria (2). Clinical use of gentamicin despite clinical benefits has been limited due to its side effects. The main side effects include liver damage that is one of the major factors of liver inefficiency in a significant number of people taking this medication. Therefore taking these medications face limitations due to the fact that one of the major side effects of Gentamicin is creating hepaotoxicity (5-3). Increased production of Reactive Oxygen Species (ROS), which can be seen after the use of gentamicin in cells, is effective in inducing toxic impacts of this drug on the structure and function of tissues (8-6).
Some studies have reported that oxidative stress has role in gentamicin-induced nephrotoxicity (9). Gentamicin enhanced the production of superoxide anion, hydrogen peroxide and hydroxyl radicals by mitochondria (10). Free radicals cause Peroxidation of phospholipids membrane, DNA strand breakage, protein denaturation. Most significant biological damage of active metabolites is their reaction with with unsaturated lipid and so their peroxidation. This effect induces changes in membrane fluidity, thus the membrane gets permeable even to molecules as large as enzymes (11). Cinnamon is a plant with the scientific name Cinnamomum zeylanicum (C. Verum) and the generic name is Cinnamon. This evergreen tree belongs to Lauraceae family is native to Sri Lanka and southeast India. Cinnamon well as being used as flavor in foods and pastries, it has medicinal and therapeutic uses. This plant is one of the oldest medicinal plants that has been used as an important medication in traditional medicine. Different parts of this plant such as the skin has many health benefits, so that its use strengthens the heart, liver, stomach and intestines; improves kidney function and increase sexual power (12). Medicinal value of this plant is due to its volatile oil .the main componenets of this essence include Cinnamaldehyde eugenol and Safrole that has activy similar to insulin and can be useful in the treatment of diabetes (13). Also, these combinations are involved in decreasing triglycerides, cholesterol and LDL level (14). Cinnamon has anti-bacterial and anti-fungal properties (15). Cinnamon is used healing wounds and treating nausea and diarrhea (16-15). This study investigated the antioxidant properties of cinnamon in reducing hepatoxity caused by gentamicin.
Materials and Methods
The present study has been conducted experimentally and it is completely random. All ethical principles of working with experimental animals have been considered in this study. Thirty six Adult Male Wistar rats weighing 10 ± 200 g and 75 days of age were randomly divided into six groups and were housed in six cages. Length of experiment was 28 days during which the rats were kept in experiment conditions including 22 ± 2 ° C and cycle of 12 hours light and 12 hours dark. Rats ate standard food of rodent (pellete). The water was provided to them in glass bottles special for them. They were allowed to eat food and water. Their Cage was disinfected with 70% alcohol three times a week. Different doses of Cinnamon extract were prepared in physiology serum and also ampoules of 100 mg in 2 mL of gentamicin were prepared. The Samples (physiology serum, cinnamon extract and gentamicin) in a volume of 0.4ml was injected with an insulin syringe intraperitoneally each day at 9 am.
Grouping rats
Thirty six Wistar rats were randomly divided into six groups and each group were subjected to the following treatments.
Group S
Rats in this group received saline solution as solvent of cinnamon extract and gentamicin.
Group C200
Rats in this group received cinnamon extract 200mg/kg B.W.
Group G100
Rats in this group received gentamicin 100mg/kg B.W.
Group GC50
Rats in this group received Gentamicin 100mg/kg B.W with cinnamon extract 50mg/kg B.W.
Group GC100
Rats in this group received gentamicin 100mg/kg B.W with cinnamon extract 100mg/kg B.W.
Group GC200
Rats in this group received gentamicin 100mg/kg B.W with cinnamon extract 200mg/kg B.W.
The animals were anesthetized 28 days after being weighed and blood samples were taken from their heart; and their serums were removed. In Serum, activity of Pyruvate Transaminase enzymes (PT), exhalo state Transaminase (OT), alkaline phosphatase (ALP) and also Albumin protein and total protein was measured.
Liver biopsy was performed and after tissue processing, the tissue section slides were prepared and stained with eosin – hematoxylin and examined by light microscopy.
Results were calculated as mean ± SD and for comparison between treatments One-way analysis of variance (ANOVA) followed by Duncan’s test for multiple comparisons between different groups were used. Significance level was considered as 0/05> P. for Data analysis and statistical tests SPSS software, edited version 22 was used and diagrams were drawn using Excel software.
Results
The results obtained from experiments are shown in diagrams. In these diagrams various groups were compared regarding parameters under study. The letters above columns in the diagrams is comparison of the significance of changes in various factors of groups. The means in columns that have at least one common letter have no significant differences with each other in P < 0/05. Figure 1 shows activity of exhalosate transaminase enzyme in the various groups. OT activity in group G showed a significant increase compared to group S. in groups GC50, GC100 and GC200 that received different doses of gentamicin along with the Cinnamon, enzyme activity was reduced and this reduction was significant in GC100 and GC200 group compared group G (0/05> P).
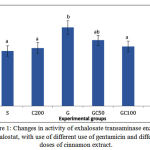 |
Figure 1: Changes in activity of exhalosate transaminase enzyme exhalostat, with use of different use of gentamicin and different doses of cinnamon extract.
|
As it is shown in Figure 2, PT Activity in Group G showed a significant increase compared to S group. in groups GC50, GC100 and GC200 that along with gentamicin, received various doses of cinnamon extract, enzyme activity was reduced and this reduction in GC200 group was significant compared to group G (0/05> P).
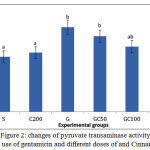 |
Figure 2: changes of pyruvate transaminase activity with use of gentamicin and different doses of and Cinnamon.
|
ALP activity in group G showed a significant increase compared to groupS. in groups GC50, GC100 and GC200 that along with gentamicin, received various doses of cinnamon extract, enzyme activity was reduced, but the rate of decline was not significant compared to group G(0/05> P) (Fig. 3).
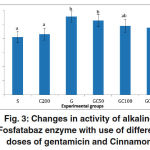 |
Figure 3: Changes in activity of alkaline Fosfatabaz enzyme with use of different doses of gentamicin and Cinnamon.
|
A sit is shown in Figure4, the amount of albumin in group G showed a significant decrease compared to groupS. in groups GC50, GC100 and GC200 that along with gentamicin received different doses of cinnamon extract, albumin increased and this rise was significant in G C100 and G C200 group compared group G(0/05> P).
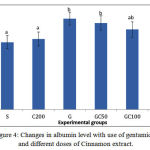 |
Figure 4: Changes in albumin level with use of gentamicin and different doses of Cinnamon extract.
|
Protein showed a significant decrease in G group compared to S group. in groups GC50, GC100 and GC200 ,that along with the Cinnamon received different doses of gentamicin, the protein content increased and this increase was significantly higher in group GC200 compared to group G (0/05> P) (Figure 5).
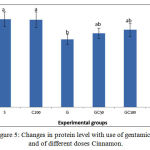 |
Figure 5: Changes in protein level with use of gentamicin and of different doses Cinnamon
|
The figure below shows photomicrograph of live tissueslices in group (S) that received only physiologyserum.
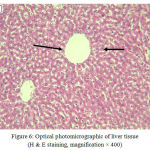 |
Figure 6: Optical photomicrographic of liver tissue (H & E staining, magnification × 400)
|
Normalliver tissue without any sign of necrosis(control group S).
Hepatocytes (right arrow), centrilobular vein (left arrow).
The results of tissue cut shows that group (S), which received physiology serume Hepatics retain their natural shape and no lymphocytic infiltration and tissue destruction was observed.
The Figure below shows liver tissue slices in group(C200) that received the cinnamon extract as 200mg/kg BW.
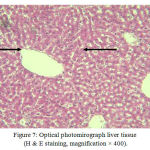 |
Figure 7: Optical photomirograph liver tissue (H & E staining, magnification × 400).
|
Normal liver tissue without any sign of necrosis (Group C200).
Hepatocytes (right arrow), centrilobular vein (left arrow) also, in group (C200) that received cinnamon extract alone, hepatocytes retain their natural form.
The Figure below shows photomicrograph of liver tissue slices (G) that used gentamicin as 100mg/kg BW.
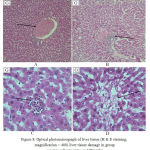 |
Figure 8: Optical photomicrograph of liver tissue (H & E staining, magnification × 400) liver tissue damage in group receiving Gentamicin as 100mg/kg
|
A: hyperemia and congestion of centrilobular vein.
B: lymphocytic infiltration around the portal space.
C: lymphocytic infiltration of tissues
D: tissue destruction and irregularity of liver hepatocytes
In Group (G), that received gentamicin alone, tissue destruction along with hyperemia and congestion of centrilobular vein (Part A), lymphocytic infiltration around the portal space (Part B), lymphocytic infiltration of tissues (Part C) and tissue destruction and irregularity of liver hepatocytes (Part D) is observed.
Figure below shows photomicroghraph of liver tissue slices in group (GC200) that received cinnamon extracts gentamicin as 200mg/kg BW.
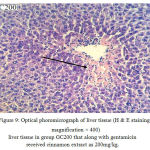 |
Figure 9: Optical phoromicrograph of liver tissue (H & E staining, magnification × 400) liver tissue in group GC200 that along with gentamicin received cinnamon extract as 200mg/kg.
|
Decrease of necrosis region, congestion and accumulation of inflammatory cells. In Group (GC200) that along with gentamicin, received 200 mg per kg body weight cinnamon extract, their problems were slightly elevated compared to group (G) and in the area of necrosis, congestion and accumulation of inflammatory cells reduced.
Discussion
gentamicin is one antibiotic that is used a lot. This Drug is used for the treatment of gram-negative bacterial infection in humans andanimals. Important complication gentamicin includes liver toxicity(hepatotexicity) (2-3). Inthis study,effect of cinnamon extra ctongentamicin-induced side effects on reducing rat liver was examined. Hepatocytes are complex metabolical liver cells that contain large amounts of enzymes. These enzymes are poured into the plasma due to liver damage, and can be useful for the detection and determination of liver damage. Exhalostatetransaminase(OT)is microsomi enzyme that is found in large quantities in the liver and is released in the blood.
Pyruvatetransaminase(PT)is a liver-specific enzymes that exists incytosol and it is indicator of liver cells as a more sensitive and specific criterion and for liver cell damage. Alkaline phosphatase is present in most tissues and increases as a result of liver diseases. Of course Depending on the type of liver disease, increase of the activity of these enzymes has different values (19-17).
In group that received Cinnamon extract (C200) compared to (S) which received physiology serum no significant difference was observed in the activity of liver enzymes including PT, OT, and ALP and also in albumin and protein content. This suggests that in normal conditions, cinnamon extract does not have significant effect on the general condition and in this specific study on the liver. In Group (G) that received gentamicin, compared to (S) which received physiology serum, activity of enzymes OT, PT, and ALP were significantly improved.
Followed by that albumin and total protein decreased and this could be indicative of liver injury induced by gentamicin (5). Since albumin and other proteins are produced primarily in the liver, followed by liver injury induced by gentamicin, albumin and other proteins also declined (5). In Groups GC50, GC100 and GC200, that along with different doses of gentamicin received different doses of cinnamon extract, roughly proportional to cinnamon extract intake, the damage caused by gentamicin decreased and activity of liver anzymes declined compared to group (G) and in Group GC200 got close to almost normal levels.
After improvement made, albumin and other proteins also increased. The conducted research indicates antioxidant compounds in cinnamon (20). Researchers know cinnamon antioxidant effect associated with two compounds of eugenol and methyl hydroxy Chalcone. Oral use of eugenol caused glutathione peroxidase activity to be normal and resotred glutathione in normal cells to increase. (21). Due to the adverse effects of free radicals and reactions of oxidative stress, presence of the body’s antioxidant compounds that can protect from damage caused by oxidative stress seems necessary. Antioxidants have special role in the prevention and treatment of diseases (23-22). It is also observed in the tissue slices slides and healing effects can be seen in group GC200 group compared to the G100. Therefore, the adverse effects caused by gentamicin in rats receiving cinnamon extract can be attributed to the antioxidant property of cinnamon extract.
References
- Ayatollahi J(2005) Evaluation of knowledge and activities of medical students in the last two years of their education about chemoprophylaxis following contact with infectious diseases. IJCID 9(26):54-9
- Stojiljkovic N, Stoiljkovic M (2006) Micromorphological characteristics of the liver and biochemical analyses in the blood of rats treated by gentamicin and verapamil. Acta medica Medianae 45(2):5-9
- Masakazu K, Yoshiko E, Masashi E (2014) Acquired resistance of Listeria monocytogenes in and escaped from liver parenchymal cells to gentamicin is caused by being coated with their plasma membrane. Microbes and Infection 16(3):237-243.
- Nayma S, Sadia C S, M Tanveer H P, Jesmine A(2012) Effects of Ashwagandha (Withania somnifera) Root Extract On Some Serum Liver Marker Enzymes (AST, ALT) In Gentamicin Intoxicated Rats 7(1):1-7
- Stojiljkovic N, Stoiljkovic M (2006) Micromorphological and histochemical characteristics of a rat’s liver treated with gentamicin. Acta medica Medianae 45(3): 24-28
- Wojciech L, Vincent LP (2005) Ternary Complexes of Gentamicin with Iron and Lipid Catalyze Formation of Reactive Oxygen Species. Chemical Research in Toxicology 18(12):357-364
- Erin E. Battin J(2009) Sulfur and selenium: A Review of reactiveOxygen species scavenging, glutathione peroxidase and metal-binding antioxidant mechanisms. Cell Biochem Biophys 55:1-23
- Jeffrey WC, Roger GU, Philip GL, Clay TC (1988) Acute Hepatocellular Effects of Erythromycin, Gentamicin, and Trospectomycin in the Perfused Rat Liver: Lack of Correlation between Lamellar Body Induction Potency and Cytotoxicity. Pharmacology & Toxicology 62(5):337-343
- Yu W, Hirotsugu I, Takahito I, Naoko H, Masaya Y (2004) Fetal cells in mother rats contribute to the remodeling of liver and kidney after injury. Biochemical and Biophysical Research Communications 325(3):961-967
- Yang C, Du X, Han Y (1995) Renal cortical mitochondria are the source of oxygen free radicals enhanced by gentamicin. Ren Fail 17:21-26
- May YH, Jochen S (1990) Formation of a cytotoxic metabolite from gentamicin by liver. Biochemical Pharmacology 40(11):R11-R14
- Inoue S, Kawanishi S (1995) Oxidative DNA damage induced by simultaneous generation of nitric-oxide and superoxide. FEBS lett 371: 86-88
- Noorolahi Z, Sahari MA, Barzegar M, Doraki N, Naghdi BH (2013) Evaluation Antioxidant and Antimicrobial Effects of Cinnamon Essential Oil and Echinacea Extract in Kolompe. J Med Plant 12(4): 14-28
- Anderson RA, Broadburst CL, Polansky MM, Schmidt WF, Khan A (2004) Isolation and characterization of polyphenol type- A polymers from cinnamon with Insulin-like biological activity. Journal of Agricultural and Food Chemistry 52(1): 65-70
- Khan A, Sfdar M, Ali Khan MM, Khattak KN, Anderson RA, (2003) Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care 26(12): 3215-3218
- Nir Y, Potasman I, Stermer E, Tabak M, Neeman I (2000) Controlled trial of the effect of cinnamon extract on Helicobacter pylori. Helicobacter 5(2): 94-97
- Kamath JV, Rana AC, Chowdhury AR (2003) Pro-healing effect of Cinnamomum zeylanicum bark. Phytotherapy Research 18(8):970-972
- Moselhy S, Ali H (2009) Hepatoprotective effect of Cinnamon extracts against carbon tetrachloride induced oxidative stress and liver injury in rats. Biol Res 42: 93-98
- Taher M, Ghannadi A, Karimiyan R (2007) Effect of volatile oil extracts of Anethumgraveolens l and Apiumgraveolens on activity of liver enzymes in rat. J Qazvin univ med sci 11(2):8-12
- Kadkhodaee M, Khastar H, Arab HA, Ghaznavi R, Zahmatkesh M (2007) Antioxidant vitamins preserve superoxide dismutase activities in gentamicin induced nephrotoxicity. Transplant Proceed 39:864-865.
- Onderoglu S, Sozer S, Mine Erbil K, Ortac R, Lermioglu F (1999) The evolution of long-term effects of cinnamon bark and olive leaf on toxicity induced by streptozotocin administration to rats. Journal of Pharmacy and Pharmacology 51:1305-1312
- Van kampen EJ. and Zijlstra WG (1985) Determination of hemoglobin and it’s derivatives. Clinical Chemistry 8:1414-18
- Peng J, Gones GL, Watson K (2000) Stress protein as biomarkers of oxidative stress: Effects of antioxidant supplement. Free Radic. Biol. Med 28:1598- 606.







