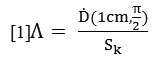Manuscript accepted on :
Published online on: 18-12-2015
Plagiarism Check: Yes
Mansour Zabihzadeh1,2*, Ali Yadollahpour1 and Leila Kargar1
1Department of Medical Physics, School of Medicine, Jundishapur University of Medical Sciences, Ahvaz, Iran. 2Department of Radiation Oncology, Golestan Hospital, Jundishapur University of Medical Sciences, Ahvaz, Iran.
DOI : https://dx.doi.org/10.13005/bpj/404
Abstract
The present study examines the effects of dose variation on brachytherapy treatment with Ir-192 source in the presence and absence of tissue heterogeneities.The effect of source location and distance between the heterogeneity and the source on dose distribution were evaluated using Monte Carlo simulation (MCNP4C code). Soft tissue heterogeneity showed no significant effect on dose distribution. Increasing the distance between the source and the phantom center, decreases dose delivery because of reduced scattering. Despite the surrounding heterogeneities, Ir-192 source can alter dose distribution in comparison with the homogeneous water phantom.
Keywords
Dose distribution; High-dose rate (HDR)brachytherapy Iridium-192 source; Tissue heterogeneities; Monte Carlo simulation
Download this article as:| Copy the following to cite this article: Zabihzadeh M, Yadollahpour A, Kargar L. The Effects of Tissue Heterogeneitieson Dose Distribution of Iridium-192 Source in Brachytherapy Treatments. Biomed Pharmacol J 2013;6(2) |
| Copy the following to cite this URL: Zabihzadeh M, Yadollahpour A, Kargar L. The Effects of Tissue Heterogeneitieson Dose Distribution of Iridium-192 Source in Brachytherapy Treatments. Biomed Pharmacol J 2013;6(2). Available from: http://biomedpharmajournal.org/?p=2688 |
Introduction
Radiotherapy is a selective method for cancer treatment via teletherapy, brachytherapy or a combination of both approaches. The ultimate goal of radiotherapy is to deliver the maximum dose to tumor tissuewhile minimizing the dose to normal tissue. Brachytherapy provides greater dose uniformity in the target volume compared with teletherapy. Due to the sharp drop in dose caused by low-energy radioactive source used in brachytherapy according to the inverse square of the distance (1/l2) law and embedding these sources within or near the target volume, the maximum dose is absorbed in the target tissue (tumor) due to its small size. With increasing the distance, the dose reached to surrounding healthy tissues is reduced drastically.
Recently, the use of Iridium-192 (Ir-192) source in high dose rate (HDR) remote (After Loading) brachytherapy is increasing because of the ability to reduce the treatment time, more control over the dose delivery process and the ability to provide safer radiation protection. Accordingly,because of the high dose delivered per treatment session and a small necessary fraction in treatments, it is important to ensure the accuracy of the dose received by target tumor tissues and the surrounding healthy tissue. Nowadays, the neededdose in brachytherapy treatments is often calculated based on the protocol proposed by The American Association of Physicists in Medicine(AAPM, TG43) in which the dose is measured in a homogeneous water phantom (1).
The potential drawback of AAPM, TG-43 protocol is the failure to take into account the effect of tissue heterogeneities (bone, air, etc.) on dose distribution. The use of dosimetry data of AAPM, TG-43 protocol (1) assuming the equality of water and various tissuesregardless of weakening and scattering effects due to heterogeneitiesmay lead to inaccurate estimates of dose for different tissue in treatment planning systems (TPS).
According to Meigooni and Nath(1992), the impact of a relatively polystern heterogeneity in solid water is more significant for sources with low energy photons. In addition,dose turbulence increases with reducing energy and increasing heterogeneity thickness(2). The results of Williamson et al. (1993) indicate that the heterogeneity correction factor varies in the range of 60-100% for a constant thickness of heterogeneity depending on the heterogeneous material, radiation source and the distance from the radiation source (3).
Kirov et al. (1996) found that for a HDR Ir-192 brachytherapy source, the dose reduction behind a tungsten alloy disk is about 2 times a steel disk. Reducing the disk diameter up to 6mm and increasing the distance between the source and detector up to 7 cm will increase the dose transfer by about 25% and 20%, respectively (4). Das et al. (1997) examined the validity of Monte Carlo simulations in vicinity of Iodine-125 in the presence of heterogeneity. The results showed that the dose distribution around a low-energy brachytherapy source like Iodine-125 is sensitive to the changes in tissue composition (5).
Daskaloy et al. (1998) proposed an analytical model to examine the effect of tissue and applicator heterogeneitiesin brachytherapy treatment planning. The proposed model is applicable for a broad range of energies used in brachytherapy (25 to 662KeV)as well as a wide range of materials (with atomic numbers of 13 to 82) withdensities in the range of aluminum (2.7 g/cm3) to platinum (21.45 gr/cm3). The heterogeneity correction factor varies from 0.09 to 0.72for heterogeneous materials. In the proposed model,the heterogeneity correction factor is underestimated when the scattering is higher,while it is overestimated when absorption is higher (6). Dos and colleagues (2006) embedded cork sheets (density: 0.25 g/cm3) within a polysternphantom to study the effect of lung heterogeneity. They concluded that the measured heterogeneity correction factor is depending on distance and energy. For example, at a distance of 2 cm from the source for quite heterogeneous conditions, the heterogeneity correction factor for Cesium-137 and Iridium-192 is 3.5 and 8.5, respectively(7).
Poon and Verhaegen (2008) proposed an analytical equation to estimate the effect of shielded anatomical heterogeneitiesand aspects of illness in HDR brachytherapy with Iridium-192 source. The proposed equation accurately estimates the effects of tungsten shields and anatomical heterogeneity for the rectum(8). Kwan et al. (2009) examined the assumption of homogenous water-equivalent rectumof unlimited size as well asthe effect of empty or filled rectal cavity on the dose absorbed on the rectal wallusing Monte Carlo simulation. The results showed that the dose in the rear wall of the rectal cavity is 22%-26% higher than the dose measured in a filled rectal cavity (9).
Graf et al. (2010) examined the effect of material heterogeneity on dosimetry in different breast brachytherapytreatments in comparison with TG-43 formula near the skin. The results showed that the dose in heterogeneous case varies up to 10% for distances greater than 2 mm from water-air interface. At distances less than 2 mm from the air-water interface, the dose difference was about 30% compared withthe homogeneous case(10).
The dosimetry of brachytherapy requires very accurate small dosimeters because of the sharp dose gradients(better resolution for dose reporting,especially in the sharp drop dose region). On the other hand,dosimetry in heterogeneous areasis so complicated in practice. Thus, the present study employs Monte Carlo simulation (MCNP-4C) to calculate the dosimetry parameters of commonly usedIridium-192 source and to precise estimate the effects of tissue heterogeneities on dose distribution. Mont Carlo simulation has extensive capabilities in the transport of photons and different particlesas well as definition of various geometries and materials.
According to the proposed assumption in AAPM, TG43, the dosimetry parameters and resulting dose distribution in a homogeneous water phantom are used to design common brachytherapy treatments. The present study aims to examine this assumption through calculating the dose distribution in the presence and absence of heterogeneities by Monte Carlo simulations. As a typical clinical brachytherapy treatment in the esophagus region, the dose distribution around the Ir-192 source embedded in esophagus was calculated due to the presence of cervical vertebrae and the spinal cord as well as trachea vicinity as air heterogeneity. The dose distribution was compared with the case in which esophagus phantom is made of water.
Methods
Monte Carlo simulation (MCNP-4C code) was used to investigate the effect of tissue heterogeneities on dose distribution. Due to high capability to define complex geometries and different materials (especially body tissues) as well as a wide variety of cross sections for different radiations, MCNP code is appropriate for dosimetric assessments,especially in the field of radiotherapy. MCNP code gives transport of various types of radiation (photons, electrons, and neutrons) in different materials along with a variety of statistical analyzes, useful outcome and the history of these particles through generating random numbers and complex physical relations governingradioactive collisions.
Due to the increasing application of Iridium-192 source in HDR brachytherapy treatment, the Ir-192 source (192Ir microSelectron (HDR) model) was used in the present study (11). The source consisted of an active Ir-192 core (half-life: 73.82 days) with a density of 22.4 gr/cm3, 0.25 cm length and 0.03 cm diameter. The core is shielded with stainless steel (1% Si, 2% Mg, 10% Ni, 19% Cu and 68% Kr) with a density of8.02 g/cm3, 0.445 length and 0.055 diameter (Figure 1). The outer radius of distal curvature is0.055. The source was placed along the y-axis. The overall length of source and leadingwirewas 5 cm. The average gamma energy emitted by Ir-192 was 0.380 MV. Allowing for scattering, the phantom dimensions were 30 × 30 × 30 cm3. The active core of the source was placed in the center of phantom.
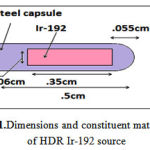 |
Figure 1: Dimensions and constituent materials of HDR Ir-192 source
|
The dose rate constant (Λ) according to AAPM, TG-43 is as follows [1]:
In which![]() is dose rate at distance of 1cm from the source at an angle of 90 ° to the source axis. Placing the source in the center of a homogeneous water phantom, the dose rate on the transverse axis at a distance of 1 cm from the source was calculated. According to TG-43 protocol, the air Chroma power, Sk, is calculated as follows:
is dose rate at distance of 1cm from the source at an angle of 90 ° to the source axis. Placing the source in the center of a homogeneous water phantom, the dose rate on the transverse axis at a distance of 1 cm from the source was calculated. According to TG-43 protocol, the air Chroma power, Sk, is calculated as follows:
In which, ![]() is air Chroma rate and d is the radial distance from the source. The source was placed in the center of a vacuum phantom with a radius of 101 cm. The Chroma rate,
is air Chroma rate and d is the radial distance from the source. The source was placed in the center of a vacuum phantom with a radius of 101 cm. The Chroma rate, ![]() , was calculated in spheres containing air with a radius of 0.25 cm at a distance of 2 cm to 100 cm on transverse axis.
, was calculated in spheres containing air with a radius of 0.25 cm at a distance of 2 cm to 100 cm on transverse axis.
To study the effect of heterogeneity on the dose distribution, bone with a density of1.92 gr/cm3, lung:0.26 gr/cm3, air: 0.00119 gr/cm3 and soft tissue with a density of 1.06 gr/cm3 were used as shown in Figure 2. The heterogeneities with a volume of 1 × 2 × 1.5 cm3 were placed on the transverse axis at a distance of 0.5, 1, 2 and 3 cm from the source.
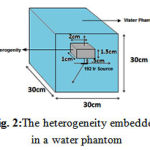 |
Figure 2:The heterogeneity embedded in a water phantom
|
To examine the effect of scattering and position of the source on the resulting dose distribution, the ratio of dose at 0.25, 0.5, 1, 2, 3, 4 and 5 m of the water phantom surface (ρ=1 gr/cm3) to the corresponding dose for the case in which the sourcecenter is located in the center ofhomogeneous water phantomwas calculated on the axis perpendicular to the central axis (Figure 3). Dose from beta radiation was not simulated because of absorption by shields which largely reduced the simulation time. The dose was calculated on spherical voxels with a radius of 0.25 on transverse axis up to 8 cm.
 |
Figure 3: Ir-192 source at different lateral distances from the phantom surface
|
To examine the effect of trachea heterogeneity (as air cavity),and cervical spine on the dose distribution of the cervical esophagus in HDR brachytherapy with Ir-192 source and to estimate the dose received by at-risk organs such as the spinal cord and thyroid, the neck phantom was simulated as a cylinder with a length of 15 cm and a radius of 5.4 cm (Figure 4). The thickness of neck skin was 0.2 cm. The cylindrical esophagus of muscle type with a length of 5 cm, 1 cm ID and a thickness of 0.3 cm was considered.The air-filled cylindrical trachea with a length of 5 cm, 1.2 cm ID and 2 cm OD with a distance of 0.3 cm from the skin surface was considered in the front of esophagus.
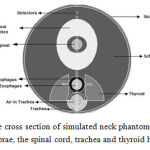 |
Figure 4: The cross section of simulated neck phantom including cervical vertebrae, the spinal cord, trachea and thyroid heterogeneities
|
Cervical spine was simulated as a cylinder with 4 cm ID, 5 cm OD with a distance of 0.65 from esophagus. The spinal canal was simulated as a cylinder with 1 cm ID and 2 cm OD in the center of cervical spine. Spinal cord was simulated as a cylinder with 1 cm ID in the center of spinal canal. Thyroid with a length of 5 cm and 1.7 cm width was placed on both sides of trachea and esophagus at a depth 1.4 cm. The source was placed in the center of esophagusso that the central axis of the source was located on longitudinal axis of esophagus. The dose was calculated on spherical voxels with a radius of 1 cm on the transverse axis of the sourceup to the skin surface. Six voxels with a radius of 0.1 cm were placed in the thyroid and the dose in voxels was calculated. The results were compared with the case of the water phantom.
Cutoff energy for photon and electron transport was assumed at 10 and 100 Kev, respectively. The number of photon used to transport gamma emitted from the source was 109. This reduces calculation error to an acceptable level of <5%(12, 13).
Results
A dose-rate constant, Λ, of 1.126 cGy/u was obtained for HDR microSelectronIr-192 source in which 1u=1μGyh-1m2.The error rate for the calculated dose distribution for points close to the source up to 3 cm was 0.09%. The error rate for the distances up to 11.805was 0.3%.
Air heterogeneity
By placing the air heterogeneity at adistance of 0.5, 1, 2 and 3 cm from the source, the ratio of dose in air heterogeneity tothe corresponding value in water heterogeneity was obtained equal to 0.95, 0.95, 0.96 and 0.96, respectively (Figure 5). After air heterogeneity, the dose increasedby 9.11-10.20%, 9.11-10.00%, 8.62-10.08% and 8.5-10.07%, respectively.
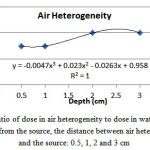 |
Figure 5: The ratio of dose in air heterogeneity to dose in water at a certain distance from the source, the distance between air heterogeneity and the source: 0.5, 1, 2 and 3 cm
|
Bone heterogeneity
The ratio of dose in bone heterogeneity to the corresponding value in water at a distance of 0.5, 1, 2 and 3 from the source was equal to 0.91, 0.98, 1.04 and 1.09, respectively (Figure 6). After the bone heterogeneity, the dose decreasedby 12-13%, 5.45-7.45%, 5.75-7.52% and 5.48-7.46%, respectively.
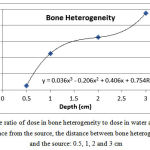 |
Figure 6: The ratio of dose in bone heterogeneity to dose in water at a certain distance from the source, the distance between bone heterogeneity and the source: 0.5, 1, 2 and 3 cm |
Lung heterogeneity
The ratio of dose in lung heterogeneity to the corresponding value water at a distance of 0.5, 1, 2 and 3 from the source was equal to 0.89, 0.95, 0.96 and 0.96, respectively (Figure 7). After the lung heterogeneity, the dose increasedby 0.40-0.98%, 6.73-8.06%, 6.39-8.10% and 6.22-8.01%, respectively.
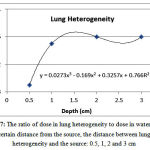 |
Figure 7: The ratio of dose in lung heterogeneity to dose in water at a certain distance from the source, the distance between lung heterogeneity and the source: 0.5, 1, 2 and 3 cm
|
Soft tissue heterogeneity
The ratio of dose in soft tissue heterogeneity to the corresponding value in water at a distance of 0.5, 1, 2 and 3 from the source was 0.85, 0.91, 0.92 and 0.92, respectively (Figure 8). The dose after the soft tissue heterogeneity at a distance of 0.5 cm from the source is 5.66-6.24% lower than the corresponding dose in water. At distances of 1, 2, 3 cm, the dose behind soft tissue heterogeneity is approximately 0.3-0.6%, 0.2-0.4% and 0.2-0.4%greater than the corresponding dose in water. Given the minimum error of 0.3,the dose increase is not significant.
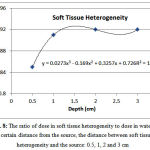 |
Figure 8: The ratio of dose in soft tissue heterogeneity to dose in water at a certain distance from the source, the distance between soft tissue heterogeneity and the source: 0.5, 1, 2 and 3 cm
|
The effect of phantom size on dose distribution
The effect of scattering caused by dispersing material on the dose rate was studied through placing the source at a distance of 0.25, 0.5, 1, 2, 3, 4 and 5 cm from the phantom surface. The results are shown in Figure 9. For example, when the source is located at a distance of 1 cm from the phantom surface the dose is decreased about 0.04-1.10% at a distance of 1 to 2 cm compared to the case in whichthe source is located in the center of phantom. Dose reduction for a distance of 3-8 cm was 2.35%-4.40%. The results show that the more distance between the source and the phantom surface, or higher distance between the source and the center of the phantom, results in lower dose at various distances compared to the case where the source is located in the center of phantom.
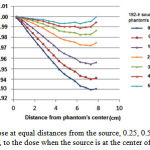 |
Figure 9: Dose at equal distances from the source, 0.25, 0.5, 1, 2, 3, 4 and 5 cm, to the dose when the source is at the center of phantom
|
As shown in Figure 10, brachytherapy with Ir-92 source delivers the maximum dose to esophagus. Then, the dose decreases with increasing the distance from the source.
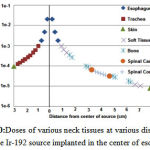 |
Figure 10:Doses of various neck tissues at various distances from the Ir-192 source implanted in the center of esophagus |
Discussion
Based on Table 1, the dose rate constant, Λ, calculated for HDR microSelectronIr-192 source in the present study is consistent with results of previous studies. Therefore, materials, components and physical parameters of the source for the transport of photons emitted from the source have been properly defined.
Table 1: Dose rate constant, Λ, for HDR microSelectron Ir-192 source
| (cGy/u)Dose Rate constant | Reference |
| 1.116 | P. Karaiskos and A. Angelopoulos (11) |
| 1.131 | Russel and Anhesjo (20) |
| 1.126 | Present Study |
Yang et al.(2011) found that the maximum dose ratio of bone and lung heterogeneity to water at a distance of 2 cm from the source is 1% and 0.99%, respectively(14). The ratio of dose for bone and lung heterogeneities in the present study was 1.04 and 0.96%, respectively showing a good agreement with the results of Yang et al. (14). As shown in Fig. 6, as the distance between bone heterogeneity and source increases, the dose increases,while the dose ratio behind the bone is lower than the corresponding value in water. However, the distance increase behind bone heterogeneity resulted in less dose reduction than water.
The average dose after bone heterogeneity is less than the corresponding dose in water. This result can be justified,because bone has a higher effective atomic number than water due to constituents with higher atomic numbers. Therefore, the probability of the photoelectric effect in bone is higher than water. As a result, it will receive more doses. The slight dose increase may be due to the factthat most collisions are of Compton type taking into consideration the average energy emitted from Ir-192 source (about 380KeV). Accordingly, it is expected that the water would receive more doses due to higher electron density compared to the bone.
However, the occurrence of the photoelectric effect releases more energy compared to Compton and the probability of occurrence of this phenomenon in the bone is higher than water. So the resultant energy absorbedby bone in this energy range is greater than water. The beam intensity decreases behind the bone due to shielding effect of the bone.Therefore dose reduction behind the bone is expected. The increase inthe distance between the radiation source and bone heterogeneity influences on dose increase within heterogeneity and behind bone heterogeneity through reducing the average beam energy.
Terribiliniet al. (2007) placedheterogeneities at a distance of1 cm from the source. The dose behind air heterogeneity was 7% higher than corresponding value in water, while the corresponding dose for bone heterogeneity was 4% lower than that of water (15). According toChandolaet al.(2010), in a similar situation, the corresponding dose behind heterogeneities was respectively 5.5-6.5% higher and 4.5-5% lower than the corresponding value in water phantom(16). In the present study, the corresponding doses behind air and bone heterogeneities were respectively 9-10% higher and 5.45-7.45% lower than doses in water phantom. These are consistent with the results of previous studies.
The discrepancies are mainly due to the differences in the size of heterogeneities in various studies. This is why the size of heterogeneityimpacts on the dose received by the rear area. In general,the dose in lung heterogeneity is lower than the corresponding dose in water because of lower lung density. The dose after lung heterogeneity is higher than the corresponding dose in water because of the increased beam intensity reached behind the lung due to lower weakening. In addition, as the distance from the source increases, the dose in lung heterogeneity increases and then reduces. According to the results, with placement of heterogeneous lung tissue near the Ir-92source, the error in predicting the dose will increase assuming a water phantom. Taking into account the actual composition of lung instead of air can lead to more accurate estimates of the dose.
With increasing distance from the source, the dose difference obtained assuming a homogeneous water phantom in AAPM, TG43 protocol reduced compared to the soft tissue (1). However, taking into account soft tissue instead of water near the source will result in lower doses.
When the source is located at a distance of 1 cm from the phantom surface, a dose reduction of approximately 2-3.5% and 4-16% at 1-2 cm and 3-8 cm was respectively observed compared to the case where the source is located in the center of the phantom. A dose reduction of approximately 0.04-1.10% and 2.35-4.40% was found in the present study. Although the received dose decreaseswith increasing the distance from the phantom center, the discrepancies are mainly due to difference between the size of heterogeneitiesand the different source type used in the two studies (16). According to Fig. 9, as the distance between the source and the phantom surface decreases, the dose decreases due to scattering. Meanwhile, to place a source at a certain distance from the source,as distance from the sourceincreases, the scattering effect due to increased dispersing material becomes more significant.
In the case of brachytherapy dosimetry process based on AAPM-TG43 protocol, to obtain the dose distribution in a water phantom, the phantom size must be large enough to produce a perfect scattering pattern (1, 17). In clinical cases where brachytherapy sources are implanted in regions close to the body surfacesuch as brachytherapy of breast, uterine wallas well as surface planting on the lip, nose and etc., the lack of dispersing material will lead to a lower dose during treatment compared with the dosimetric parameters obtained through placement of source placed in the center of the water phantom. Therefore, the dose correction factors (<1)should be applied for more accurate dose delivery in clinical cases.
Compared with homogeneous neck phantom, air (trachea) and bone (cervical vertebrae) heterogeneities have no effect on dose distribution. But the dose delivered to organs at risk such as thyroid and spinal cord was 13% and 5/5% lower than the corresponding doses in the water phantom. Therefore, the dose of organs at risk in the conventional treatment planning software (based on dose distribution in homogeneous water phantom) is overestimated than Monte Carlosimulation(18, 19). Dose of the cervical spine is approximately 1.3% higher than the corresponding dose in water phantom which is not significant.
According to the results of the present study,assuming a homogeneous water phantom in dosimetry of radioactive sources used in brachytherapy based on common AAPM-TG43 protocol due to the lack of consideration of heterogeneities such as bone and lung can ultimately leads to dose overestimation and underestimation respectively within and behind bone and estimating lower and higher doses respectively within and behind lung tissue (1). The probability of photoelectric effect is directly related to the third power of the atomic number and reciprocal of the third power of energy. Thus, it is of great importance to include more accurate composition of tissues for dose estimation, especially in dosimetry of low-energy brachytherapy sources like Iodine-125 (28.5 KeV) and Pd (20.8 KeV),especially at close distances. It is recommended that the effect of heterogeneities on dose estimation, especially in the vicinity of source is considered in future treatment planning software according to AAPM, TG43 protocol. It is also suggested that the necessary correction factors are applied for an accurate estimation of the dose.
Acknowledgments
This study was performed as a Master thesis at the Department of Medical Physics, Ahvaz Jundishahpur University of Medical Sciences and was financially supported by the research project No. 90243-U.
References
- Rivard MJ, Coursey BM, DeWerd LA, Hanson WF, Huq MS, Ibbott GS, et al. Update of AAPM Task Group No. 43 Report: A revised AAPM protocol for brachytherapy dose calculations. Medical physics. 2004;31:633.
- Meigooni AS, Nath R. Tissue inhomogeneity correction for brachytherapy sources in a heterogeneous phantom with cylindrical symmetry. Medical physics. 1992;19:401.
- Williamson JF, Perera H, Li Z, Lutz WR. Comparison of calculated and measured heterogeneity correction factors for 125I, 137Cs, and 192Ir brachytherapy sources near localized heterogeneities. Med Phys. 1993;20(1):209-22.
- Kirov A, Williamson J, Meigooni A, Zhu Y. Measurement and calculation of heterogeneity correction factors for an Ir-192 high dose-rate brachytherapy source behind tungsten alloy and steel shields. Medical physics. 1996;23:911.
- Das PK, Keleti D, Zhu Y, Kirov AS, Meigooni AS, Williamson JF. Validation of Monte Carlo dose calculations near< sup> 125</sup> I sources in the presence of bounded heterogeneities. International Journal of Radiation Oncology* Biology* Physics. 1997;38(4):843-53.
- Daskalov GM, Kirov AS, Williamson JF. Analytical approach to heterogeneity correction factor calculation for brachytherapy. Medical physics. 1998;25:722.
- Das I, Both S, Cheng C. Inhomogeneity Correction in Brachytherapy. Med Phys. 2006;33(6):1.
- Poon E, Verhaegen F. An Analytical Approach to Account for Shielding, Anatomical Heterogeneities and Patient Dimensions for 192Ir High Dose Rate Brachytherapy Applications. . Med Phys 2008;35(6):1.
- Kwan I, Wilkinson D, Cutajar D, Lerch M, Rosenfeld A, Howie A, et al. The effect of rectal heterogeneity on wall dose in high dose rate brachytherapy. Medical physics. 2009;36:224.
- Graf M, Scanderbeg D, Yashar C, Jiang S. Monte Carlo Dose Comparison Assessing Material Inhomogeneity Effects in Breast Brachytherapy. Med Phys 2010;37(6):1.
- Karaiskos P, Angelopoulos A, Sakelliou L, Sandilos P, Antypas C, Vlachos L, et al. Monte Carlo and TLD dosimetry of an Ir high dose-rate brachytherapy source. Medical physics. 1998;25:1975.
- Rivard MJ, Beaulieu L, Mourtada F. Influence of Material Heterogeneities for Brachytherapy Dose Calculations. Med Phys. 2010;37(6):1.
- Russel KR, Ahnesjo A. Dose calculation in brachytherapy for a192Ir source using a primary and scatter dose separation technique. . Phys Med Biol. 1996;41(6):24.
- Yang Y, Rivard MJ. Evaluation of brachytherapy lung implant dose distributions from photon-emitting sources due to tissue heterogeneities. Medical physics. 2011;38:5857.
- Terribilini D, Manser P, Frei D, Volken W, Mini R, Fix M, editors. Implementation of a brachytherapy Ir-source in an in-house system and comparison of simulation results with EGSnrc, VMC++ and PIN. Journal of Physics: Conference Series; 2007: IOP Publishing.
- Chandola R, Tiwari S, Kowar M, Choudhary V. Effect of inhomogeneities and source position on dose distribution of nucletron high dose rate Ir-192 brachytherapy source by Monte Carlo simulation. Journal of cancer research and therapeutics. 2010;6(1):54.
- Granero D, Perez-Calatayud J, Pujades-Claumarchirant M, Ballester F, Melhus CS, Rivard MJ. Equivalent phantom sizes and shapes for brachytherapy dosimetric studies of Ir and Cs. Medical physics. 2008;35:4872.
- Duzenli C, McClean B, Field C. Backscatter into the beam monitor chamber: implications for dosimetry of asymmetric collimators. Med Phys. 1993;20(2 Pt 1):363-7.
- Cheong K, Lee M, Kang S, Park S, Kim K. A Monte Carlo study for evaluation of tissue heterogeneity effect of inversely optimized intracavitary high dose rate brachytherapy plan. . Med Phys. 2009;36(6):1.
(Visited 1,022 times, 1 visits today)