Manuscript accepted on :
Published online on: 18-12-2015
Plagiarism Check: Yes
Ndubuisi N. Nwobodo
Department of Pharmacology and Therapeutics, Faculty of Clinical Medicine, Ebonyi State University, Abakaliki, Nigeria.
Corresponding Author E-mail: nnwobodo@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/415
Abstract
Sepsis is a complex inflammatory syndrome induced by infection triggering the activation of multiple humoral cascades. A number of therapeutic interventions aimed at blocking these pathways have been carried out. However, merely blocking one component of inflammation is insufficient to arrest the multiple activated inflammatory cascades. The 3-HMG-CoA reductase inhibitors (statins) exhibit pleiotropic effects independent of lipid lowering, encompassing antiinflammatory, immunomodulatory, antiapoptic and antithrombotic properties. Evidence from experimental models of sepsis and randomized clinical trials suggest that statins have potential and promising prospects in ameliorating the consequences and reducing the mortality and morbidity associated with severe sepsis. Notwithstanding, the crucial role of statins in the prevention and treatment of sepsis, there is need for caution among clinicians, in the use of statins , considering the adverse effects associated with statin use in critically ill patients who may be on other medications.
Keywords
Experimental model; Inflammatory syndrome; Pleiotropic effect; Sepsis; Statins; Therapeutic implication
Download this article as:| Copy the following to cite this article: Nwobodo N. N. Statins and Sepsis: Possible Connections and Therapeutic Implications. Biomed Pharmacol J 2013;6(2) |
| Copy the following to cite this URL: Nwobodo N. N. Statins and Sepsis: Possible Connections and Therapeutic Implications. Biomed Pharmacol J 2013;6(2). Available from: http://biomedpharmajournal.org/?p=2723 |
Introduction
Sepsis is generally defined as the systemic inflammatory response syndrome that is induced by infection and may eventually lead to organ dysfunction1. A number of therapeutic interventions such as low dose corticosteroids and activated protein C, have been employed with a view of improving outcome and survival in sepsis2, yet mortality continues to increase. A delicate balance exists between widespread triggering of defence mechanism by invading micro-organisms and both direct/indirect effects of the micro-organisms and their products. It has been revealed in a retrospective study, that patients with bacteremia concomitantly treated with HMG-CoA reductase inhibitors (statins) showed decreased overall mortality compared to control not treated with statins3. Sepsis activates multiple complex steps in the inflammatory cascade and a number of experimental and clinical trials have focused on agents aimed at blocking these steps4-7. It should be noted that in severe sepsis syndrome, blockade of single component of the inflammatory cascade is insufficient and may not have any impact.
The pleiotropic effects of statins independent of lipid lowering encompasses antiinflammatory, inmmunomodulatory, antiapoptic, antioxidant, antiproliferetive, antithrombotic and endothelium protective features8. There is substantial evidence in favour of the fact that statin therapy may be beneficial in prevention and treatment of sepsis9,10. This review paper explores the pathophysiology of sepsis, the impact of statin therapy on outcome of sepsis and the mechanisms involved.
Pathophysiology of Sepsis
The localization and control of bacterial invasion, while initiating tissue repair is the normal response to infection. However, the generalisation of response to infection and involvement of normal tissues distant from original site of infection, invariably leads to sepsis. Innate immune cells recognize and bind to microbial components thereby initiating host response to infection. This may occur by recognition and binding of pattern recognition receptors (PRRs) on the surface of host immune cells to the pathogen associated molecular patterns (PAMPs) of the invading microorganism11. Sepsis can be regarded as a complex syndrome involving activation of a variety of systems. The outcome in the case of sepsis is dependent on the delicate balance between the pro-inflammatory and anti-inflammatory mediators12. It has been reported that significant changes occur at multiple levels within both the coagulation system and the cellular regulators during sepsis13. The inflammatory response represents an important component of sepsis as elements of this response drive the physiological processes which translate into systemic inflammatory response syndrome (SIRS). The concept of SIRS recognizes that lethally altered pathophysiology could exist without necessarily, presence of positive blood culture14. Sepsis is assumed to be driven by hyper-inflammatory response. This concept is driven by the fact that septic patients at increased risk for death have increased levels of tumour necrosis factor (TNF)15. Tissue injury and widespread inflammatory alterations similar to observation in septic patients occur following injection of TNF into laboratory animals16,17. Similar observations have led to institution of clinical trials with a view of blocking TNF18,19. The above individual trials did not show significant improvement in sepsis survival. Notwithstanding, a meta-analysis of all the TNF inhibitors revealed improvement in overall outcome20. Inflammatory response in sepsis is heterogeneous and not clearly defined as some patients may benefit from enhancing the inflammation whereas others will be better served by diminishing the inflammatory response. Individualized therapy in sepsis is critical, as evidence from preclinical model of sepsis indicate that diminishing inflammatory responses may be beneficial in animals at high risk of morbidity21.
Cellular dysfunction in sepsis may manifest as either depressed function or excessive activation. Excessive activation occurs as a result of damage to nearby cells by neutrophils generating excess toxic products22. Neutrophil failure to phagocytize and clear invading pathogens is an example of depressed cellular function. Endothelial cells are dysfunctional in sepsis, due to increased expression of adhesion molecules on the endothelial cells, resulting in white blood cells being attached. Endothelial cells elaborate anticoagulant molecules such as protein C. The remarkable relationship between the inflammatory and coagulation systems in sepsis has become increasingly evident23. A state of net immune suppression known as compensatory anti-inflammatory response syndrome (CARS) is attained as sepsis and inflammation progress24.
Role of Statins in Sepsis
A number of therapeutic approach has been employed to modulate immunological response and the delicate balance between pro-inflammatory and anti-inflammatory responses in sepsis. The 3-HMG-CoA reductase inhibitors, known as statins, a class of cholesterol lowering drugs reputed for their pleiotropic effects independent of cholesterol lowering are reported to play a crucial role in prevention and treatment of sepsis. Recent report from clinical trials provide new data on the use of statins for treating sepsis25. Evidence from animal models of sepsis indicate that statin use may prevent and modulate sepsis severity26,27. Reducing rates of sepsis-related mortality among patients on statins and a decrease in the severity and incidence of sepsis among patients treated with statins have been noted in two observational human studies3,28. A decrease in the incidence of sepsis among patients treated with statins hospitalized for a cardiovascular event was reported in a population based cohort study29. Another study reported that reduction in the risk of hospitalization for sepsis in patients with severe kidney disease receiving dialysis was strongly and independently associated with statin use30. Immune response to infection is modulated by statins, decreasing the risk of sepsis in infected patients. The outcome of studies on the effects of statins on human immunodeficiency virus, yeast and salmonella suggests that statins may also have antimicrobial effects31-34. Sepsis induced coagulopathy may be blunted by the antithrombotic effects of statins35,36. Data from experimental models of sepsis suggest that statins markedly decreased nitric oxide (NO) production8,26. Simvastatin reduced nitric oxide production and reverted the impaired vascular responsiveness in a pre-treatment rat model26. The inducible heat shock and cytoprotective protein, hemeoxygenase (HO-1) is involved in attenuating endothelial dysfunction consequent on sepsis. It has been shown that the anti-proliferative, anti-inflammatory and anti-oxidant properties of simvastatin may be largely attributed to hemeoxygenase (HO-1) induction37,38. Statins attenuate hemodynamic perturbation due to myocardial dysfunction in sepsis. Report from an experimental model, revealed mean survival time close to four times that in untreated control mice pretreated with simvastatin39. Same study also confirmed complete preservation of hemodynamic status and cardiac function39. A similar study noted that survival time was markedly improved with statin treatment initiated 6hours after induction of sepsis40. A study recommended the conduct of large scale clinical trials aimed at evaluating the precise role of statins in the primary prevention and therapy of sepsis41.
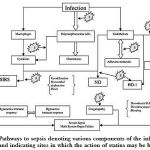 |
Figure 1: Pathways to sepsis denoting various components of the inflammatory cascade and indicating sites in which the action of statins may be beneficial. |
(Adapted and modified from-Novack V., Terblanche M. and Almog Y. Do statins have a role in preventing or treating sepsis? Crit Care. 10(1): 113 (2006).
Conclusion
Statins have a crucial role to play in the prevention and treatment of sepsis as a result of their well known pleiotropic effects independent of lipid lowering. However, there is still need for caution among clinicians in translating these outstanding research findings into routine clinical practice; considering the adverse effects, particularly rhabdomyolysis and hepatic toxicity associated with the use of statins, moreso, in critically ill patients who may be receiving other medications.
References
- Bone R.C., Balk R.A., Cerra F.B., Delinger R.P., Fein A.M., Knaus W.A., Schein R.M. and Sibbald W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 101: 1644–1655 (1992).
- Riedemann N.C., Guo R.F. and Ward P.A. Novel strategies for the treatment of sepsis. Med. 9: 517–524 (2003).
- Liappis A.P., Kan V.L., Rochester C.G. and Simon G.L. The effect of statins on mortality in patients with bacteremia. Infect. Dis. 33: 1352–1357 (2001).
- Bone R.C., Fisher C.J. Jr, Clemmer T.P., Slotman G.J., Metz C.A. and Balk R.A. A controlled clinical trial of high dose methyl-prednisolone in the treatment of severe sepsis and septic shock. Engl. J. Med. 317; 653–658(1987).
- Annane D. Corticosteroids for septic shock. Care Med. 29: S117–S120 (2001).
- Bernard R., Vincent J.L., Laterre P.F., LaRosa S.P., Dhainaut J.P., Lopez-Rodriguez A., Steingrub J.S., Garber G.E., Helterbrand J.D., Ely E.W. and Fisher C.J. The recombinant human activated protein: efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 344: 699-709 (2001).
- Ziegler E.J., Fisher C.J. Jr., Spring C.L., Stranbe R.C., Sadoff J.C., Foulke G.E., Wortel C.H., Fink M.P., Dellinger R.P. and Teng N.N. Treatment of gram negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin: a randomized double blind placebo-controlled trial. The HA-1A Sepsis Study Group. Engl. J. Med. 324: 429 – 436(1991).
- Blanco-Colio L.M., Tunon J., Martin-Ventura J.L. and Egido J. Anti-inflammatory and immunomodulatory effects of statins. Kidney Int. 63: 12-23 (2003).
- Martin G.S., Mannino D.M., Eaton S. and Moss M. The epidemiology of sepsis in the United States 1979 through 2000. Engl. J. Med. 348: 1546–1554 (2003).
- Almog J. Statins, inflammation and sepsis: hypothesis. 124: 740–743 (2003).
- Cinel I. and Dellinger R.P. Advances in pathogenesis and management of sepsis. Opin. Infect. Dis. 20(4): 345-352 (2007).
- Jacobi J. Pathophysiology of sepsis. J. Health Syst. Pharm. 59(Suppl 1): S3–S8 (2002).
- Esmon CT, Interactions between inflammation and coagulation. J. Haematol. 131: 417–430 (2005).
- American College of Chest Physician/Society of Critical Care Medicine Consensus Conference. Definition of sepsis for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 20: 864–874(1992).
- Waage A, Halstensen A. and Espevik T. Association between tumor necrosis factor in serum and fatal outcome in patients with meningoccal disease. Lancet. 1: 355–357 (1987).
- Tracey K.J., Bentler B., Lowry S.F., Merryweather J., Wolpe S., Milsark I.W., Hariri R.J., Fahey T.F., Zentalla A., Albert J.D., Shires G.T., Cerami A. Shock and tissue injury induced by recombinant human cachectin. 234: 470–474(1986).
- Remick D.G., Kunkel R.G., Larrick J.W. and Kunkel S.L. Acute in vivo effects of human recombinant tumor necrosis factor. Invest. 56: 583–590.
- Bentler B., Milsark I.W. and Cerami A.C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. 229: 869-871(1985).
- Remick D.G. Cytokine therapeutics for the treatment of sepsis: why has nothing worked? Pharm. Des. 9: 75–82 (2003).
- Marshall J.C. Such stuff as dreams are made of: mediator-directed therapy in sepsis. Rev. Drug Discov. 2: 391–405 (2003).
- Remick D.G., Bolgos G.E. and Siddiqui J. Inflammatory status in sepsis alters efficacy of interleukin–18 binding protein therapy. Care Med. 31: 2096–2101 (2003).
- Weiss S.J. Tissue destruction by neutrophils. Engl. J. Med. 320: 365–376 (1989).
- Abraham E. Coagulation abnormalities in acute lung injury and sepsis. J. Respir. Cell Mol. Biol. 22: 401–404 (2000).
- Ward N.S., Casserly B. and Ayala A. The compensatory anti-inflammatory response syndrome (CARS) in critically ill patients. Chest Med. 29(4): 617-625 (2008).
- Kruger P., Bailey M., Bellomo R. et al. A multicenter randomized trial of atorvastatin therapy in intensive care patients with severe sepsis. J. Respir. Crit. Care Med. 187: 743–750 (2013).
- Giusti-Paiva A., Martinez M.R., Felix J.V., da Rocha M.J., Carnio E.C., Elias L.L. and Antunes-Rodriques J. Simvastatin decreases nitric oxide overproduction and reverts the impaired vascular responsiveness induced by endotoxic shock in rats. Shock. 21: 271–275 (2004).
- Ando H., Takamura T., Ota T., Nagai Y. and Kobayashi K. Cerivastatin improves survival of mice with lipopolysaccharide–produced sepsis. Pharmacol. Exp. Ther. 294: 1043–1046 (2000).
- Almog Y., Shefer A., Norack V., Maimon N., Barski L., Eizinger M., Friger M., Zeller L. and Danon A. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation. 110: 880–885 (2004).
- Hackam D.G., Mamdani M., Li P. and Redelmier D.A. Statins and sepsis in patients with cardiovascular desease: population-based cohort analysis. Lancet. 367: 413–418 (2006).
- Gupta R., Plantinga L.C., Fink N.E., Melamed M.L., Coresh J., Fox C.S., Levin N.W. and Powe N.R. JAMA. 297(13): 1455–1464 (2007).
- del Real G., Jimenez-Baranda S., Mira E., Lacalle R.A., Lucas P., Gomez-Monton C., Alegret M., Pena J.M., Rodriquez-Zapata M., Alvarez-Mon M., Martinez A.C. and Manes S. Statins inhibit HIV-1 infection by down regulating Rho activity. Exp. Med. 200: 541–549 (2004).
- Giguere J.F. and Tremblay M.J. Statin compounds reduce human immunodeficiency virus type1 replication by preventing the interaction between virion-associated host intercellular adhesion molecule-1 and its natural cell surface ligand LFA-1. Virol. 78: 12062–12065 (2004).
- Catron D.M., Lange Y., Borensztajn J., Sylvester M.D., Jones B.D. and Haldar K. Salmonella enterica serovar typhimurium requires nonsterol precursors of the cholesterol biosynthetic pathway for intracellular proliferation. Immun. 72: 1036–1042 (2004).
- Ikeura R., Murakowa S. and Endo A. Growth inhibition of yeast by compactin (ML–236B) analogues. Antibiot. 41: 1148–1150 (1988).
- Steiner S., Speidl W.S., Pleiner J., Seidenger D., Zorn G., kaun C., Wojta J., Huber K., Minar E., Woltz M. and Kopp C.W. Simvastatin blunts endotoxin-induced tissue factor in vivo. Circulation. 111: 1841-1846 (2005).
- Dangas G., Badimon J.J., Smith D.A., Unger A.H., Levine D., Shao J.H., Meraj P., Fier C., Fallon J.T. and Ambrose J.A. Pravastatin therapy in hyperlipidemia: effects on thrombus formation and the systemic hemostatic profile. Am. Coll. Cardiol. 33: 1294–1304 (1999).
- Tee T.S., Chang C.C., Zhu Y., Shyy J.Y. Simvastatin induces heme-oxygenase-1: a novel mechanism of vessel protection. Circulation. 110: 1294–1302 (2004).
- Grosser N., Hemmerle A., Berndt G., Erdmann K., Hinkelmann U., Schroder S., Wijayanti N., Immenschuh S. and Schroder H. The antioxidant defense protein hemeoxygenase-1 is a novel target for statins in endothelial cells. Free Radic. Biol. Med. 37(12): 2064–2071 (2004).
- Merx MW, Liehn E.A., Janssens U., Lutticken R., Schrader J., Hanrath P. and Weber C. HMG-CoA reductase inhibitor simvastatin profoundy improves survival in a murine model of sepsis. Circulation. 2004; 109(21): 2560–2565 (2004).
- Merx M, Liehn E, Graf J., van de Sandt A., Schaltenbrand M, Schrader J, Hanrath P, Weber C. Statin treatment after onset of sepsis in a murine model improves survival. 112(1): 117-124 (2005).
- Novack V., Terblanche M. and Almog Y. Do statins have a role in preventing or treating sepsis? Care. 10(1): 113 (2006).
(Visited 171 times, 1 visits today)







