Manuscript accepted on :
Published online on: 16-12-2015
Plagiarism Check: Yes
Z. Myrkhalykov, Z. Konarbayeva, A. Saparbekova*, L. Mamaeva, A. Yussubaeva and M. Auezov
South Kazakhstan State University, Shymkent 160012, Kazakhistan.
DOI : https://dx.doi.org/10.13005/bpj/400
Abstract
Twenty-one strains of various lactic acid bacteria (LAB) were isolated from raw dairy materials in South Kazakhstan. In the present study of coagulation time, moisture retention, aroma, and proteolytic and lipolytic activities, we report the identification of Streptococcus lactis SSà-1 and S. cremoris K-3 as the best strains for serving as starter cultures in the production of dairy products. Increasing amounts of germinated wheat introduced into the fermentation cultures accelerated milk coagulation and increased the viscosity of clots formed by the action of lactic acid. Germinated and shredded wheat at a concentration range of 3%–5.5% optimally stimulated bacterial growth in whole milk. We also determined that the addition of whey proteins (3%–5%), which are rich in essential amino acids, significantly stimulated the growth of LAB.
Keywords
Lactic acid bacteria; Acid formation; Germinated wheat; Whey proteins; Sour milk products
Download this article as:| Copy the following to cite this article: Myrkhalykov Z, Konarbayeva Z, Saparbekova A, Mamaeva L, Yussubaeva A, Auezov M. Selection of Starter Cultures for Producing Milk Products in Media Containing Grain Additives. Biomed Pharmacol J 2013;6(2) |
| Copy the following to cite this URL: Myrkhalykov Z, Konarbayeva Z, Saparbekova A, Mamaeva L, Yussubaeva A, Auezov M. Selection of Starter Cultures for Producing Milk Products in Media Containing Grain Additives. Biomed Pharmacol J 2013;6(2). Available from: http://biomedpharmajournal.org/?p=2670 |
Introduction
Among microorganisms used for food production, lactic acid bacteria (LAB) occupy a special position. The selection of starter cultures plays an important role in the efficient production of sour milk products and in obtaining high yields. For this purpose, LAB have been characterized in great detail, particularly for manufacturing specific products. The most important properties of LAB for producing foods and medically useful products are related to consistency, taste, and aroma (Pridannikova, 2001).
Worldwide, scientists are engaged in exploiting LAB for manufacturing traditional sour milk products to enhance nutrition as well as the function of the immune system. Advances in our understanding of the physiology, biochemistry, molecular biology, and genetics of LAB have greatly expanded their application for generating products that enhance health and nutrition (Almagambetov, 2001; Saubenova et al., 1988; Latov et al., 1985; Fuller, 1994; Mȕller et al., 1995; Montville and Chen, 1998). Investigators have particularly focused on characterizing LAB present in various natural sources to obtain strains that best suit a particular process. These efforts have resulted in various products such as medicines and foodstuffs. The lifestyles of the residents of large cities have resulted in the so-called “syndrome of megalopolis” that manifests as metabolic disorders, immunodeficiency, and adverse changes in the microbial ecology of the digestive tract. With respect to the latter, the levels of bifidobacteria and LAB, which act as probiotics in the gastrointestinal tract, have diminished, whereas those of potential microbial pathogens have increased. Thus, more attention has been focused on the development of lactic acid products and probiotics that comprise a variety of microorganisms. First, the interaction of microbes in their natural habitat and, above all, the symbiosis of separate strains are considered.
In Kazakhstan, 75%–90% of the population has been reported to experience disbacteriosis of the normal gut flora (Kushugulova et al., 2006). Therefore, it is important develop nutritional interventions to prevent and treat enteric disease. The present study aimed to characterize naturally occurring LAB species with respect to their growth and metabolic properties to enhance the production of nutritional supplements and medicines for addressing this important public health issue.
Materials and Methods
Sources of bacteria: LAB were isolated from lactic acid-containing foods and other sources from cities (Shymkent, Turkestan, Kentau, and Zhetysai) and villages (Akbulak, Karasu, Martobe, Sairam, Koksayek, Chubar, Kazygurt, Tassay, Kershetas, and Azatlyk) in Southern Kazakhstan.
A number of media are recommended for isolating LAB from various sources. These include liquid and solid media that contain amino acids and vitamins, yeast extracts (Yeastrel) and autolysates, and protein hydrolysates. Starter cultures that contain different species will also be formulated with the expectation that the component strains will act synergistically. Isolation of LAB included the following steps: selection of local milk containing LAB, inoculation of the enriched culture on a medium rich in nutrients, and incubation at an optimum temperature. Once axenic cultures were obtained, subclones were isolated by culturing them on sterile non-fat milk, and their species was identified. The isolates were then characterized with respect to their ability to ripen milk, produce acid, inhibit the growth of other bacteria, and resist antibiotics. The isolates were subjected to microscopy, and their ability to coagulate milk and their organoleptic properties were studied. Strains that generated products with a viscous, creamy consistency resulting from high moisture retention were selected.
Moisture retention of the clots generated by LAB was determined by centrifugation at 1000. For this purpose, 10 mL of the destroyed clot was introduced into a 15-mL plastic centrifuge tube and then centrifuged at 1000 for 5 min. The whey was decanted into either a graduated glass centrifuge tube or a graduated beaker to measure the volume, and the results were expressed as mL whey/10-mL clot. The proteolytic activity of the cultures was determined by the content of tyrosine produced by incubation with non-casein whey. High activity was defined as 300 µg/mL, average as 200–300 µg/mL, and low as <200 µg/mL. Table 3 presents the results for aroma production and proteolytic and lipolytic activities. The levels of lactic acid produced by the cultures were determined using the Turner method (Technical specifications 10-02-02-789-65-97, 1991; Krus and Shalygina, 2000).
Table 1: Antimicrobial activity of lactic acid bacteria
| Strain | Diameter of zones of growth suppression (mm) | ||||
| Salmonella typhimurium | Escherichia coli | Pseudomonas aeruginosa | Staphylococcus aureus | Bacillus subtilis | |
| S. cremoris SL-12 | 6 ± 0.5 | 10.7 ± 0.5 | 13.5 ± 0.5 | 14.6 ± 0.5 | 6.3 ± 0.5 |
| S. lactis SH-4 | 9.2 ± 0.5 | 6 ± 0.5 | 12.6 ± 0.5 | 16.6 ± 0.5 | 7.4 ± 0.5 |
| L. аcidophilus SHА-2 | 13.0 ± 0.5 | 12.6 ± 0.5 | 16.3 ± 0.5 | 18.8 ± 0.5 | 26.3 ± 0.5 |
| S. lactis SN-1 | 12.3 ± 0.5 | 4.7 ± 0.5 | 8.5 ± 0.5 | 14.6 ± 0.5 | 8.6 ± 0.5 |
| S. thermophilus IТ-2 | 14.4 ± 0.5 | 11.6 ± 0.5 | 14.4 ± 0.5 | 15.6 ± 0.5 | 18.4 ± 0.5 |
| L. аcidophilus Тr-2 | 11.0 ± 0.5 | 12.6 ± 0.5 | 7.3 ± 0.5 | 24.6 ± 0.5 | 11.5 ± 0.5 |
| S. thermophilus Тr-3 | 18.7 ± 0.5 | 4.7 ± 0.5 | 8.5 ± 0.5 | 14.6 ± 0.5 | 8.6 ± 0.5 |
| S. lactis Тr-4 | 8.4 ± 0.5 | 11.6 ± 0.5 | 14.4 ± 0.5 | 8.6 ± 0.5 | 18.4 ± 0.5 |
| S. lactis SS -2 | 11.7 ± 0.5 | 19.8 ± 0.5 | 11.3 ± 0.5 | 24.6 ± 0.5 | 11.5 ± 0.5 |
| S. cremoris SSp-3 | 8.4 ± 0.5 | 10.7 ± 0.5 | 12.5 ± 0.5 | 14.6 ± 0.5 | 6.3 ± 0.5 |
| S. lactis SSа-1 | 19.2 ± 0.5 | 17.6 ± 0.5 | 16.2 ± 0.5 | 15.1 ± 0.5 | 17.7 ± 0.5 |
| S. thermophilus SPа-1 | 9.2 ± 0.5 | 6 ± 0.5 | 12.6 ± 0.5 | 16.6 ± 0.5 | 7.4 ± 0.5 |
| L. bulgaricus SPm-2 | 4.0 ± 0.5 | 11.6 ± 0.5 | 6.3 ± 0.5 | 10.8 ± 0.5 | 6.2 ± 0.5 |
| S. lactis SPz-1 | 12.3 ± 0.5 | 4.4 ± 0.5 | 9.5 ± 0.5 | 14.7 ± 0.5 | 8.6 ± 0.5 |
| L. plantarum SPz-2 | 10.5 ± 0.5 | 13.8 ± 0.5 | 10.4 ± 0.5 | 8.9 ± 0.5 | 9.4 ± 0.5 |
| S. lactis K-1 | 12.7 ± 0.5 | 8.7 ± 0.5 | 9.5 ± 0.5 | 14.6 ± 0.5 | 8.6 ± 0.5 |
| S. cremoris K-3 | 18.9 ± 0.5 | 20.2 ± 0.5 | 14.4 ± 0.5 | 21.6 ± 0.5 | 13.4 ± 0.5 |
| L. plantarum Kz-2 | 11.7 ± 0.5 | 9.8 ± 0.5 | 11.3 ± 0.5 | 8.6 ± 0.5 | 15.5 ± 0.5 |
| S. thermophilus Kz-4 | 10.5 ± 0.5 | 10.7 ± 0.5 | 7.5 ± 0.5 | 14.4 ± 0.5 | 6.8 ± 0.5 |
| S. lactis ТU-5 | 15.0 ± 0.5 | 21.6 ± 0.5 | 6.8 ± 0.5 | 10.8 ± 0.5 | 6.2 ± 0.5 |
| S. lactis Тuz-2 | 10.7 ± 0.5 | 12.7 ± 0.5 | 11.5 ± 0.5 | 14.7 ± 0.5 | 11.6 ± 0.5 |
StrainDiameter of zones of growth suppression (mm)Salmonella typhimuriumEscherichia coliPseudomonas aeruginosaStaphylococcus aureusBacillus subtilisS. cremoris SL-126 ± 0.510.7 ± 0.513.5 ± 0.514.6 ± 0.56.3 ± 0.5S. lactis SH-49.2 ± 0.56 ± 0.512.6 ± 0.516.6 ± 0.57.4 ± 0.5L. аcidophilus SHА-213.0 ± 0.512.6 ± 0.516.3 ± 0.518.8 ± 0.526.3 ± 0.5S. lactis SN-112.3 ± 0.54.7 ± 0.58.5 ± 0.514.6 ± 0.58.6 ± 0.5S. thermophilus IТ-214.4 ± 0.511.6 ± 0.514.4 ± 0.515.6 ± 0.518.4 ± 0.5L. аcidophilus Тr-211.0 ± 0.512.6 ± 0.57.3 ± 0.524.6 ± 0.511.5 ± 0.5S. thermophilus Тr-318.7 ± 0.54.7 ± 0.58.5 ± 0.514.6 ± 0.58.6 ± 0.5S. lactis Тr-48.4 ± 0.511.6 ± 0.514.4 ± 0.58.6 ± 0.518.4 ± 0.5S. lactis SS -211.7 ± 0.519.8 ± 0.511.3 ± 0.524.6 ± 0.511.5 ± 0.5S. cremoris SSp-38.4 ± 0.510.7 ± 0.512.5 ± 0.514.6 ± 0.56.3 ± 0.5S. lactis SSа-119.2 ± 0.517.6 ± 0.516.2 ± 0.515.1 ± 0.517.7 ± 0.5S. thermophilus SPа-19.2 ± 0.56 ± 0.512.6 ± 0.516.6 ± 0.57.4 ± 0.5L. bulgaricus SPm-24.0 ± 0.511.6 ± 0.56.3 ± 0.510.8 ± 0.56.2 ± 0.5S. lactis SPz-112.3 ± 0.54.4 ± 0.59.5 ± 0.514.7 ± 0.58.6 ± 0.5L. plantarum SPz-210.5 ± 0.513.8 ± 0.510.4 ± 0.58.9 ± 0.59.4 ± 0.5S. lactis K-112.7 ± 0.58.7 ± 0.59.5 ± 0.514.6 ± 0.58.6 ± 0.5S. cremoris K-318.9 ± 0.520.2 ± 0.514.4 ± 0.521.6 ± 0.513.4 ± 0.5L. plantarum Kz-211.7 ± 0.59.8 ± 0.511.3 ± 0.58.6 ± 0.515.5 ± 0.5S. thermophilus Kz-410.5 ± 0.510.7 ± 0.57.5 ± 0.514.4 ± 0.56.8 ± 0.5S. lactis ТU-515.0 ± 0.521.6 ± 0.56.8 ± 0.510.8 ± 0.56.2 ± 0.5S. lactis Тuz-210.7 ± 0.512.7 ± 0.511.5 ± 0.514.7 ± 0.511.6 ± 0.5
Results and Discussion
We successfully isolated 21 strains of LAB, which included members of the families Streptococcaceae and Lactobacillaceae. The isolates were identified as follows: Streptococcus lactis, SH-4, SN-1, Tr-4, SS-2, SSа-1, SPz-1, K-1, ТU-5, Tuz-2; S. cremoris, SL-12, SSp-3, K-3; S. thermophilus, IТ-2, Tr-3, SPa-1, Kz-4; Lactobacillus аcidophilus, SHА-2 and Tr-2; L. bulgaricus, SPm-2; and L. plantarum, SPз-2 and Kz-2 (isolated from wheat grains).
Viable microorganisms such as LAB present in food and nutritional supplements can produce antibiotics that can suppress undesirable microflora present in the gastrointestinal tract. Table 1 presents studies of antimicrobial activities of LAB. L. аcidophilus SHА-2, S. thermophilus IТ-2, S. lactis SS-2, S. lactis SSа-1, S. cremoris K-3, and S. lactis Tuz-2 showed the highest levels of antimicrobial activities, suggesting that they may be useful in the manufacture of sour milk products as dietary supplements and therapeutics. The coagulation activity and organoleptic properties of the isolates are the most important and decisive indicators that determine their suitability for the manufacturing process. Strains with high organoleptic indicators and physicochemical properties that correspond to those of the end product, including the fermentation rate (using a 5% starter culture), average acidity (within the limits of 70–90 °Т), hyperviscosity (>5 Pa × s × 10−3), and sufficient water-retaining capacity, must be selected. S. cremoris SL-12, S. cremoris K-3, S. lactis SSа-1, and S. thermophilus SPa-1 met these criteria (Table 2).
Table 2: Physicochemical properties of lactic acid clots
| LAB
|
Coagulation time (h) | Titrable acidity
|
Viscosity (Pa × s × 10−3)
|
Moisture retention (mL/10 mL) |
| S. cremoris SL-12 | 5–6.5 | 70 | 5.20 | 2.3 |
| S. lactis SH-4 | 4.5–5 | 75 | 5.45 | 3.5 |
| L. аcidophilus SHА-2 | 4.5–5 | 135 | 6.05 | 4.2 |
| S. lactis SN-1 | 4.5–5 | 110 | 5.20 | 2.8 |
| S. thermophilus IТ-2 | 3.5–4.5 | 115 | 5.00 | 3 |
| L. аcidophilus Tr-2 | 4–5.5 | 120 | 5.15 | 3.3 |
| S. thermophilus Тr-3 | 4–5 | 105 | 5.20 | 3.6 |
| S. lactis Тr-4 | 3.5–4 | 90 | 4.55 | 2.6 |
| S. lactis SS-2 | 6–6.5 | 75 | 4.78 | 3.6 |
| S. cremoris SSp-3 | 3.5–4 | 85 | 5.05 | 2.5 |
| S. lactis SSа-1 | 4–5 | 75 | 5.20 | 2.5 |
| S. thermophilus SPа-1 | 4–4.5 | 90 | 5.85 | 2.4 |
| L. bulgaricus SPm-2 | 7–7.5 | 130 | 4.20 | 5.2 |
| S. lactis SPz-1 | 5–5.5 | 90 | 4.20 | 3.2 |
| L. plantarum SPz-2 | 20–21 | 135 | 3.20 | 4.5 |
| S. lactis K-1 | 4.5 | 100 | 4.20 | 3.6 |
| S. cremoris K-3 | 4.5 | 75 | 5.40 | 2.3 |
| L. plantarum Kz-2 | 25–28.5 | 145 | 3.20 | 5.3 |
| S. thermophilus Kz-4 | 5–5.5 | 110 | 4.75 | 3.8 |
| S. lactis ТU-5 | 8–8.5 | 75 | 5.40 | 3.4 |
| S. lactis Тuz-2 | 4–5 | 95 | 3.80 | 4.1 |
To develop new lactic acid combined products, aroma generation and proteolytic and lipolytic activities of LAB must be taken into account. There is a direct dependence between organoleptic properties of lactic acid products, e.g., their taste, aroma, and content of free amino acids, amino acid metabolites, free fatty acids, and mono and diacylglycerides that are generated by the proteolytic and lipolytic activites of LAB (Hammes and Tichaczek, 1994; Corsetti et al., 1996). In the presence of only lactic acid, the clots of milk are characterized by a flat flavor. The aroma of the clot is contributed by the by-products of lactic acid fermentation (carbon dioxide, acetic acid, ethanol, ethyl carbonic acid, diacetyl, and ethyl aldehyde), the products of proteolysis (peptides, amino acids, and sulfur-containing compounds such as mercaptans and dimethyl disulfite), and lipolysis (fatty acids). The acid-forming capacity of the strains differed. The limit of acid accumulation ranged from 110 °Т to 300 °Т. Milk coagulation occurred between 3–38 h.
The optimum temperature for culturing LAB differs from that required for fermentation and can vary depending on conditions. The activity of ripening process depends on the length of the lag phase of growth, and the activity of acid formation is the compulsory criterion for determining the activity of LAB. S. cremoris SL-12, S. cremoris K-3, S. lactis SSа-1, and S. thermophilus SPa-1 were used to study the rate of acid production (Fig. 1). S. cremoris K-3 and S. lactis SSа-1 exhibited the highest levels of acid production, and clots formed between 10–12 h of fermentation after inoculating 10 mL of milk with a loopful of culture. The clots were characterized by a dense, uniform consistency and had a nice taste of sour milk and a nice aroma. The number of viable microorganisms was also the highest among the isolates (Fig. 2). We therefore conclude that S. lactis SSа-1 and S. cremoris K-3 can be recommended as starter cultures for manufacturing milk products.
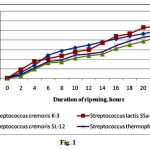 |
Figure 1: Kinetics of acid formation by lactic acid bacteria |
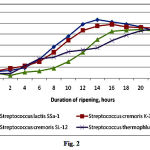 |
Figure 2: Growth of lactic acid bacteria on a 10 mL clot |
The general characteristics of the S. lactis SSа-1 strain are as follows: Gram-positive, non-motile cocci that occur as single cells (0.8–0.9 µ), in pairs, or in short chains of 2–4 cells and do not form spores. S. lactis is widely used to manufacture sour milk products and induces the formation of very dense, pricking clots (Yegorov et al., 1990). In contrast, the morphology of S. cremoris K-3 differs in that it forms short chains of 3–5 cells (0.8–1.0 µ). This strain ripened the cultures within 5–5.5 h using a 3% starter culture at a wide optimum temperature range of 22–30°C. It does not grow in media containing 4%–6% NaCl, regenerate, or coagulate litmus milk. S. cremoris K-3 inhibited the growth of other bacteria. It is resistant to infection with bacteriophages and to several antibiotics such as gentamicin and streptomycin. This isolate is also used to manufacture sour milk products that serve as dietary supplements and probiotics. S. lactis and S. cremoris are present in most milk products and play an important role in the process of ripening and formation of sour milk products. S. cremoris forms clots that resemble sour cream in consistency. Therefore, it is used to produce sour milk products that are characterized by a thick consistency.
Grain products are more frequently used as an additive in the production of sour milk products. The main purpose of grain fillers in these products is to enrich their content of vitamins, trace minerals, dietary fibers, and organic acids that stimulate the growth of LAB. The combination of plant and milk raw materials is a growing trend in the industry to produce qualitatively new food products. Grains of flour starch are crystalline and finely dispersed. Starch binds large amounts of water at ambient temperatures. Dextrins are the primary products of starch hydrolysis and are colloids that form adhesive solutions with water. Their molecular mass and properties depend on the degree of starch hydrolysis. Dextrins have a low capacity for binding water and represent approximately 3%–5% of the weight of wheat flour from germinated grain.
Flour produced from germinated wheat contains the dietary fibers pectin, lignin, cellulose, and hemicelluloses that promote the growth of LAB. Simple carbohydrates are also used to culture LAB. The ability of LAB to ferment carbohydrates and alcohols is an important diagnostic feature of these bacteria. Germinated wheat provides vitamin B that is required for the growth of LAB (Fig. 3). The proliferation of LAB increases with increasing amounts of flour derived from germinated wheat, causing increases in the rate of coagulation and in the viscosity of sour milk clots. In the present study, when clots formed in cultures containing 3% and 5% wheat flour, the numbers of bacteria were 5.6 × 107 and 2.3 × 108, respectively. In general, germinated wheat stimulates the growth of LAB in concentrations ranging from 1% to 10%, with the optimum concentration being 3%–5% (Fig. 3).
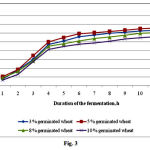 |
Figure 3: Growth of lactic acid bacteria as a function of germinated wheat concentration |
Germinated wheat enriches milk products with a number of functional ingredients, e.g., dietary fibers, oligosaccharides, minerals, unsaturated fatty acids, and a number of vitamins that supplement the original quantities of essential amino acids. The introduction of up to 10% flour from germinated wheat does not have a negative effect on the taste of milk–grain clots. This lowers the price of milk products, making them more affordable as well as facilitating the production of multifunctional products. Thus, germinated wheat may be used in the production of combined milk products as additional sources of biologically active substances such as proteins, polysaccharides, and minerals. The possibility of using grain crops to manufacture milk products balances the content of proteins, fats, and carbohydrates. This should increase the shelf life and reduce production costs.
Milk is an expensive raw material, and its manufacture is labor-intensive. This emphasizes the importance of developing more efficient and effective conversion processes. Milk whey is a secondary raw material used by the dairy industry to manufacture cheeses, cottage cheese, and casein. The production of whey protein concentrates for enriching milk–grain products is based on ultrafiltration techniques that have been applied to produce cottage cheese whey (as well as other milk raw materials), for example, and provides the ability to enrich components on the basis of the molecular mass, size, and ionic strength (Kirillova, 2005).
Table 3: Aroma production and proteolytic and lipolytic and lipolytic activities of lactic acid bacteria
| Strain | Aroma-producing capacity | Proteolytic activity, µ/10 mL | Lipolytic activity |
| S. cremoris SL-12 | high | 330 | high |
| S. lactis SH-4 | average | 200 | average |
| L. аcidophilus SHА-2 | high | 320 | high |
| S. lactis SN-1 | average | 210 | average |
| S. thermophilus IТ-2 | high | 300 | average |
| L. аcidophilus Tr-2 | high | 325 | high |
| S. thermophilus Tr-3 | average | 200 | average |
| S. lactis Tr-4 | high | 320 | high |
| S. lactis SS-2 | low | 200 | average |
| S. cremoris SSp-3 | high | 315 | high |
| S. lactis SSа-1 | high | 330 | high |
| S. thermophilus SPа-1 | average | 215 | average |
| L. bulgaricus SPm-2 | high | 320 | high |
| S. lactis SPz-1 | low | 210 | average |
| L. plantarum SPz-2 | average | 300 | high |
| S. lactis K-1 | low | 210 | average |
| S. cremoris K-3 | high | 330 | high |
| L. plantarumKz-2 | average | 225 | average |
| S. thermophilus Kz-4 | average | 315 | high |
| S. lactis ТU-5 | high | 235 | average |
| S. lactis Тuz-2 | high | 325 | high |
The main advantages of ultrafiltration techniques applied to cottage cheese whey are as follows: direct regulation of composition and other properties with comparatively low energy consumption, creation of new dairy products of high biological value with decreased caloric content, and low or no waste production. Ultrafiltration employs semipermeable membranes with pores sizes of 10–100 nm that are capable of retaining components with molecular masses ≥104 (Sataev, 2006). To study the influence of whey proteins on the growth of LAB, a membrane device with immobile membrane elements was developed in our laboratory by M.A. Concentrated cottage cheese whey protein is retained and concentrated, while salts and lactose pass into the filtrate. The proteins retain their native properties. The pressure necessary to realize the ultrafiltration process is 0.1–1 MPa (1–10 atm).
The composition of the initial product was as follows: protein, 0.6%–0.8%; fat, 0.05%–0.06%; lactose, 4.5%–5.1%; ash, 0.5%–0.6%; and dry residue, 4.2%–7.0%. The composition of the end products was as follows: whey protein concentrate; protein, 11%–15%; fat, 2.5%; lactose, 1.2%; ash, 0.15%; and dry residue, 11.5%–19%. The components of the filtrate were as follows: protein, <0.1%; fat, <0.1%; lactose, 4.0%–4.5%; ash, 0.6%–0.7%; dry residue, 3.8%–5.3%; and рН, 5.0–5.8.
The whey proteins produced using this ultrafiltration device stimulated the growth of LAB (Fig. 4). We conclude that the whey proteins provided a source of nitrogen that could be readily assimilated by the cultures because LAB lack the ability to synthesize organic nitrogen compounds and only some LAB use inorganic nitrogen to synthesize organic compounds. LAB require arginine, cysteine, glutamic acid, leucine, phenylalanine, tryptophan, tyrosine, and valine for growth. When present in optimum amounts in milk whey, they enhance the growth of LAB. Analysis of the end products of cultures containing a germinated wheat formula is presented in Table 4.
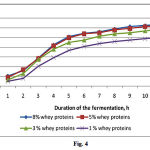 |
Figure 4: Growth of lactic acid bacteria as a function of whey protein concentration |
Table 4: Composition of the combined sour milk product
| Component | Quantity, % (by weight) |
| Fat-free milk | 80–82 |
| Germinated wheat | 3–5.5 |
| Concentrate of whey proteins | 3–5 |
| Gelatin | 1.95–2.9 |
| Sugar | 5–7 |
| Flavoring matter | 0.05–0.1 |
| Bacterial starter | 2–2.5 |
Conclusion
Here we describe the isolation of two LAB strains (S. lactis SSа-1 and S. cremoris K-3) that possess antimicrobial activity and can serve as starter cultures for producing sour milk products. The milk–grain products may be useful not only for preventive purposes but also for the treatment of many diseases, including those of the gastrointestinal tract.
Acknowledgments
This work was supported by the Agricultural Ministry of Republic of Kazakhstan and the Ministry of Education and Science of Kazakhstan. The authors thank anonymous reviewers and the editors for their objective and valuable comments related to our work.
References
- Almagambetov, K.H., 2001. Biological properties of lactobacilli. Biotechnology 1: 27-31.
- Corsetti, A., Gobbetti, M., Smacchi, E., 1996. Antibacterial activity of sourdough lactic acid bacteria: isolation of a bacteriocin like inhibitory substance from Lactobacillus sanfrancisco C57. Food Microbiol. 198 (3): 447-456.
- Fuller, R., 1994. Probiotics: an overview. In: Human health: The Contribution of Microorganisms, Gibson, S.A.W. (Ed.). Springer-Verlag, New York, USA, pp: 63-73.
- Hammes, W.P., Tichaczek, P.S., 1994. The potential of lactic acid bacteria for the production of sale and wholesome food. Z. Zebensm. Unters. Forsch. 198 (3): 193-201.
- Kirillova, A.G., 2005. Membrane technologies of APV company for milk industry. Milk Ind. 5: 52-53.
- Kushugulova, A.R., Rahimova, S.E., Bekbolatova, Zh.T., Oralbaeyva, S.S., Saduahasova, S.A., Pernekulova, A.Zh., 2006. Differentiation of probiotic microorganisms on the basis of molecular methods. Materials of scientific – practical conference of young scientists of Kazakh State medical academy, Kazakhstan, Almaty, pp: 38-39. {4.4 [EN] Missing information in reference entry}
- Latov, V.K., Gordienko, S.V., Kogan, A.S., 1985. Receiving amino acids and other physiologically active substances by complex processing of microbiological raw materials. All-Union Symposium Physico-chemical properties of biopolymers in solution and cells In the collection of scientific works: Use of Biomass of Microorganisms for Food Purposes. Russia, Pushchino, p: 46-51.
- Montville, T.J., Chen, Y., 1998. Mechanistic action of pediocin and nisin: recent progress and unresolved questions. Appl. Microbiol. and Biotechnol. 5: 511-519.
- Mȕller, T., Behrend, U., Mȕller, M., 1995. Antagonistic activity in plant-associated lactic acid bacteria: Emerging Principles and Applications. In: Microbial Physiology, and Gene Regulation, Scheffers, W.A. and van Dijken, J.P. (Eds.). Book of Abstracts, Delft University Press, The Netherlands, pp: 447-448.
- Pridannikova, A., 2001. Starter cultures for sour milk products. Milk Ind., 12: 29-30.
- Krus, G.N. and Shalygina, A.M., 2000. Research methods of milk and milk products. Kolos, Moskow.
- Sataev, M.I., 2006. Provision of ecological safety of environmental health situation by adsorption and membrane methods of purifying water and gas flows. Dissertation of Doctor of Engineering, M. Auezov South Kazakhstan State University, Kazakhstan (in Shymkent)
- Saubenova, M.G., Hmelevskaya, L.K., Konuspayeva, K.Sh. 1988. Enrichment of milk whey by protein and carotin. Abstracts of the All-Union Conference on Biotechnology of cereals. In the collection: Technology of Manufacture of Microbiological Synthesis Product. Kazakhstan, Alma-Ata, рp: 161-165.
- Technical specifications 10-02-02-789-65-97., 1991. Bacterial starters, yeast and test – cultures. Moscow, Russia.
- Yegorov, N.S., Stoyanova, L.G., Pyatnitsyna, I.N., Zadoyana, S.B., 1990. Strain of bacteria Streptococcus lactis producer of nisin, A.S. SU 1551744 Al.







