Manuscript accepted on :
Published online on: 12-12-2015
Plagiarism Check: Yes
R.Tamil Selvi1 and M. Ilavazhahan2
1Department of Chemistry, Bharthi women’s College, Chennai-600 108, India.
2Department of Advanced Zoology and Biotechnology, Sir Theagaraya College, Chennai - 600 021, India.
Abstract
Histopathological changes in the gill tissue of the fish Catla catla exposed to sublethal concentration of Methyl parathion and Ferrous sulphate was studied. Marked pathological changes are observed ranging from bulging of tips of primary gill filaments to fusion of disorganized secondary gill filaments.
Keywords
Catla catla; Methyl parathion; Ferrous sulphate; Histopathological changes; Primary gill filament
Download this article as:| Copy the following to cite this article: Selvi R. T. Ilavazhahan M. Histopathological Changes in Gill Tissue of the Fish Catla catla Exposed to Sublethal Concentration of Pesticide Methyl Parathion and a Heavy Metal Ferous Sulphate. Biomed Pharmacol J 2012;5(2) |
| Copy the following to cite this URL: Selvi R. T. Ilavazhahan M. Histopathological Changes in Gill Tissue of the Fish Catla catla Exposed to Sublethal Concentration of Pesticide Methyl Parathion and a Heavy Metal Ferous Sulphate. Biomed Pharmacol J 2012;5(2). Available from: http://biomedpharmajournal.org/?p=2514 |
Introduction
Human population growth and industrial development have been the major causes of coastal contamination around the world during recent years (Caussy et al., 2003). The subsequent accumulation of xenobiotic compounds in sediment, seawater or prey organisms has been shown to be directly linked to adverse health in both humans and fish (Ashraf, 2005).
The ecological effects of pollutants in aquatic ecosystems and their bioavailability and toxicity are closely related to species distribution, both in the solid and the liquid phase of the aquatic ecosystem. Pollutants are transferred to the plankton, aquatic plants, mollusks and fish. A wide range of microscopic and macroscopic animals and plants live in and on bottom sediments of the aquatic ecosystems, and a great number of these organisms ingest organic matter form these sediments (Mosisch and Arthington, 2001).
Fish mortality is not only caused by fish diseases but also by deterioration of water quality, industrial effluents, metallic pollutants and pesticide residues. Pesticides reach the aquatic environment by various sources like air drift and run off causing water pollution. Bio magnification of persistent pesticides brings about pathophysiological changes and mass mortalities among fish population.
Pesticides drained to the aquatic environment are primarily of agricultural origin and which may also stem from effluent from manufacturing plants. Since there is great concern about toxic hazards in the aquatic ecosystem due to pesticides, either from surface run-off from paddy fields or through direct application into ponds for the control of parasites, it is necessary to study the cellular changes in fish tissue associated with this toxicity.
In an environment, a variety of toxicants are present in different concentrations. Though the concentration of some toxicants is not lethal to the organism, they may still cause severe damages at the cellular level in different tissues. Several of the pesticide and metal pollutants in the environment are myelo and neurotoxic in nature and cause deformities to tissues like liver and kidney because of their involvement in detoxification. Neurotoxic substances affect brain, while gills being the first vital organs to take up the toxins during the process of respiration, they are more vulnerable and prone to damages.
Intensive activity in industrial and agricultural sectors has inevitably increased the levels of heavy metals in natural waters (Jordao et al., 2002). Heavy metals play a major role among pollutants of environmental concern (Singer et al., 2005). Heavy metals are serious pollutants of the aquatic environment because of their environmental persistence and ability to be accumulated by aquatic organisms (Veena et al., 1997). Rapid industrialization lead to contamination of natural waters with metals due to dumping of untreated wastes in the aquatic habitats, causing deleterious effects to fish (Javed, 2004). The metal pollutants are present in water bodies in a mixture of two or more major metals, often forming complexes which are more toxic than individual toxicants (Sujatha, 2006). Thus for healthy fish production, it is very important to evaluate the harmful effects of heavy metals known to cause instantaneous physiological disorders (Subramanian, 2004).
Histopathological biomarkers can be indicators of the effects on organisms of various anthropogenic pollutants and are a reflection of the overall health of the entire population in the ecosystem. The alterations in cells and tissues in vertebrate fish are recurrently used biomarkers in many studies. Histopathological investigations have proved to be a sensitive tool to detect direct effects of chemical compounds within target organs of fish in laboratory experiments. The degree of pathological intensity is dependent on the dose and duration of exposure. Histopathological studies have been conducted to help establish causal relationships between contaminant exposure and various biological responses. Histopathological biomarkers embody tissue lesions arising as a result of a previous or current exposure of the organism to one or more toxins.
Considerable interest has been shown in recent years in histopathological study while conducting sub-lethal tests in fish. Tissue changes in test organisms exposed to a sub-lethal concentration of toxicant are a functional response of organisms which proides information on the nature of the toxicant.
The toxicity of pollutants can be assessed by the extent of histopathological damage in the organisms and the degree of evident cell damage in relation to the concentration of pollutant employed. The fishes get their tissues easily damaged due to water pollutants, the gills and liver being the most potent sites that show changes in their histoarchitecture at very early stages of toxic stress.
Several studies have emphasized the histopathology of tissues after exposure to pollutants. Inflammatory alterations of lamellar epithelium and hyperplasia were reported in gills of freshwater carp, Cirrhinus mrigala during 48hr exposure to a sub lethal dose of malathion (Roy and Datta, 1991). Similar observations were reported in chlorpyrifos exposed fish, Cirrhinus mrigala, Tilak et al. (2005) and Babu et al. (2007) have reported fenvalerate induced changes such as epithelial hyperplasia, epithelial necrosis, desquamation and lamellar fusion besides epithelial lifting, oedema, swelling at the tip of the secondary lamellae and curling of secondary lamellae in gill tissue of freshwater fish Cirrhinus mrigala. Similar changes have been reported in Anabas testudiens exposed to paper mill effluent (Prasanta Nanda and Panigrahi, 2004). Other studies on histopathology of fish include the effect of chlorine on tilapia (Ramalingam and Murabai, 2002), antigen on rohu (Vardhni and Gowri, 2002) and tannery effluent on common carp (Rani et al., 2004). Sujatha (2006) reported damage in gill architecture in Catla catla due to the effect of heavy metals, Zinc and Copper.
The toxicity of any toxicant is either acute or chronic. The chronic studies include both histochemistry and pathology. Mode of action of different chemicals varies leading to varied effects on various body tissues. Some toxins exert their effect locally at the portal of entry resulting in damage to external surface of the body. Thus, various chemicals with their varied mode of action to different tissues thereby bringing about certain architectural changes ultimately culminating in either death of the organism or making the organism less labile for its survival.
In the present study, an attempt has been made to know the extent of damage to the general architect of gill of the test fish Catla catla under exposeure to sub-lethal concentrations of Methyl parathion and Ferrous sulphate.
Materials and Methods
Fingerlings of Catla catla of relatively same size ranging from (8 to10 cm) and weight about (5-8 gm) were collected from culture ponds of Bharath Fish Farm, Poondi, Thiruvallur district, Tamil Nadu. The fish were oxygen packed in polythene bags and brought to the laboratory with minimal stress during transit. They were released very carefully into the fish tanks half filled with bore well water. They were maintained in the stocking tank and acclimatized before experimentation.
The fish feed was prepared with sieved rice bran, pounded groundnut oil cake, tapioca powder and mineral mixture as followed by Ramaiah (1982). The fish fingerlings were fed daily with pelleted feed at 5% body weight, in two split doses, one in the morning and the other in the evening. The feeding was stopped one day prior to experiment.
The fishes were maintained in the aquarium tanks of size 1’l x 2’b x 1’h throughout the period of study. Potassium permanganate (0.02%) was used as disinfectant to clean the tanks before and after experiments. The tanks were filled with water (2 litres per fish) and covered with mesh cloth to prevent the mosquitoes breeding in the water and also to prevent the fish from jumping out of tank. The fish without any structural, behavioral and clinical symptoms were chosen for experiments, after careful observation. Fish were divided into groups of ten each and exposed to different toxicants, viz., heavy metal compound (Ferrous sulphate) and a pesticide (Methyl parathion), independently.
One fourth of LC50 values obtained from the above experiments was taken as the sub lethal concentration (SLC).
Suitable controls were maintained without toxicants and analyses were done on Zero day, 4th and 7th days of experimentation. Gill tissue from the control and 7th day experimental groups were selected for histopathological observations.
Fish were killed and the tissue (gill) were fixed in 10% neutral buffered formalin. The fixed tissues were dehydrated in an increasing gradient of alcohol (70, 80, 90, and 100%) for 30 min each and were eventually dried in acetone, and cleared in xylene for 30min. The tissues were then infiltrated by embedding in molten wax and sectioned at 8µ. The paraffin sections were then mounted on a slide, stained with haematoxylin and counterstained with eosin.
To study the pathogenicity of various toxicants, the section of tissue were observed under microscope and the condition of the tissue were photographed at lower and higher power of magnification using Nikon micro photographic equipment.
Results and Discussion
Histology is useful technique for investigating the toxic effect of various pollutants. Such a study also offers opportunity to locate the effect of pollutants in various organs and systems of animals. This type of study in fish has been to a great extent is handicapped because of the lack of adequate histological literature concerning various fish oragans (Hinton et al., 1997). Considerable interest has been shown in recent years in histopathological studies while conducting sub-lethal tests in fish. Tissue changes in test organisms exposed to sub-lethal concentration of toxicant are a functional response of organisms which provides information on the nature of toxicant.
In fish, gill is the first organ to which any pollutant comes into contact. Fish gill is very sensitive to changes in the composition of the environment and is an important indicator of waterborne toxicants. Consequently, injury to gill epithelium is a common response observed in fish exposed to a variety of contaminants. The severity of damage to the gills depends on the concentration of the toxicant and the period of exposure.
Pesticide (Methyl parathion) was taken in test dose of 1ppm to 10ppm to study the effect of pesticide toxicity in the fingerlings of Catla catla. The fish exposed to this toxicant were severely affected. Increase in opercula movement, loss of equilibrium, frequent surfacing, changes in body colour, increased secretion of mucus, irregular swimming activity, rapid jerk movement were observed. No mortality was recorded at 1ppm concentration of the pesticide. Fish survived through 48hr up to toxic concentration of 3ppm, and 50% mortality was recorded at 4.8ppm of concentration. The mortality recorded was 80% and 100% at 8 and 10ppm concentration respectively. 96hr LC50 of pesticide methyl parathion was noted as 4.8ppm by graphic method. SLC (1/4th LC50) of was calculated as 1.2ppm from the 96hr LC50 value.
The fingerlings of Catla catla were exposed to ferrous sulphate in the concentrations of 2 to 20ppm and the pathological symptoms observed. White precipitate formation was seen in experimental tanks. Loss of equilibrium, changes in opercular movement, change of orientation, erratic swimming were the other symptoms observed. No mortality was recorded in all the four days at the concentration of 2ppm. 10% mortality was noted at 4ppm on the first day of experiment. With regard to 6ppm concentration 20% mortality was observed on second day and later it reached 30%. The highest mortality of 100% was observed at 13ppm and 15ppm concentrations. 50% mortality was recorded in 8 and 9ppm concentration and 50% of mortality line intercepted the graph to show 8.4ppm as corresponding LC50 for the toxicant. SLC was calculated as 2.1ppm from the 96hr LC50 value.
The gill arches of Catla catla in the control group showed normal arrangement pattern of primary and secondary lamellae (Fig.1). The arches contain primary lamellae. Projecting on the lateral sides of primary lamellae are the secondary lamellae. The entire mass of primary lamellae is covered by stratified squamous epithelium. The surface of the secondary lamellae is covered with a delicate layer of a simple squamous epithelium that is the active exchange pillar cells. In the core of the primary lamellae is a rigid mass of cartilaginous tissues around which are traces of vascular channels. The chloride cells are more frequent at the base of the secondary lamellae.
Varied morphological changes occurred in the gill tissue of the treated fingerlings of catla and the gill exhibited marked alterations in their epithelia. The epithelium was no longer continuous, particularly the more delicate respiratory lamellae. Thus, there was fusion at adjacent secondary lamellae as a result of hyperplasia. Oedema at the secondary lamellae and swelling of the epithelia cells were observed. The pillar cells have been altered and blood spaces expanded. There is a severe hyperplasia with profound oedematous changes characterized by epithelial detachment. The elongated secondary lamellae with club-like structures were the other pathological changes observed in the histological sections of gills of the fingerlings of catla treated with two toxicants (Fig.2 &3).
The Catla fingerlings exposed to different toxicants showed an extensive damage to their gill architecture in the present study and this is in agreement with the earlier observations (Ramesh Kumar et al., 1988; Srivastava and Maurya, 1991; Sujatha, 2006). The gill is important site for the entry of heavy metals that provokes lesions and gill damage (Bols et al., 2001).
Variations in the epithelial surface of gills show important physiological adaptations relating to the area available for increased gaseous exchange (Kendall and Dale, 1979). Their vulnerability is thus considered because of their external location and for the fact that they are in intimate contact with water and are thus liable to damage by irritant materials. The lamellar reduction of the treated fish must have been caused by respiratory stress as the sum of all the physiological responses by which an animal tries to maintain or re-establish a normal metabolism in the face of a physical or chemical force. Haemorrhage and sloughing of the bronchial arteries at the opercular end of the primary lamellae can disrupt the circulation of the deoxygenated blood via the bronchial arteries into the secondary lamellae in a direction opposite to that of water flow. As a result, oxygen uptake is hampered. This can cause asphyxiation, tissue necrosis and finally death.
The severity of damage depends on the toxic potential of a particular compound or pesticide accumulated in the tissue (Jayantha Rao, 1984). A number of pathological changes have been reported in fishes exposed to different organochlorine, organophosphate and synthetic pyrethroid pesticides (Anitha Sussan , 1994, Vijayalaksmi and Tilak, 1996; Ramanakumari, 1999; Yacob,1999; Veeraiah, 2001; Tilak et al., 2001a; Tilak et al., 2001b; Tilak and Yacobu, 2002). The changes include the bulging of tips of primary gill filaments. The secondary filaments lost their original shape. The pillar cell nucleus showed necrosis and developed vacuoles in the secondary gill epithelium.
The proliferated gill lesions are often observed after exposure of fish to water soluble toxicants. The nutritional gill disease consists of lamellar epithelial hyperplasia with eventual fusion of secondary lamellae near the tips of gill filaments (Lohar, 2000). Eller (1971) described endrin induced histopathological changes in cut throat trout and reviewed the gill lesions in freshwater teleosts. The other reports on activity of different pesticides on fishes are Girija (1987), Ramamurthy (1988) and Vijayalakshmi and Tilak (1996). Wannee et al. (2002) observed filament cell proliferation, lamellar cell hyperplasia, lamellar fusion and aneurysm in the nile tilapia, Oreochromis niloticus under exposure to glyphosate for 96hr. Mazhar Sultana and Dawood Sharif (2004) reported that these pathological changes in the gills might have resulted in such a shift from aerobic to anaerobic pathway in tissues of fish under exposure to toxicity. Tilak et al., (2005) also reported on these lines in fishes exposed to different pesticides.
In the present study, it was observed that toxic exposure caused lamellar telangiectasis (clubbed appearance) along with oedema and mucoid metaplasia. The clubbed appearance of lamellae is due to lamellar hyperplasia in which cells are derived from primary lamellae and migrate to the distal end. This result in accumulation of cells at the leading edge of secondary lamella, which is colloquially called ‘clubbing’ of lamellae (Roberts and Rodger, 2001). Also, the pillar cell architecture was probably altered by increase in chloride cell number and their subsequent bulging to the surface. Mucoid metaplasia is also very distinctly observed as the entire inter lamellar space seems to be filled up by such cells. Similar observations have also been reported due to exposure to pesticide (Tilak et al., 2001) and mercury (Gupta and Rajbanshi, 1995).
Thus, when fish is happened to exposed to pesticides, they cause irrepairable architectural changes, in vital organs like gill, making the fish less fit for better survival. These histopathological changes can alter various physiological activities of fish such as release of various enzymes and consequently metabolism is effected.
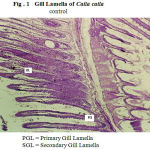 |
Figure 1: Gill Lamella of Catla catla.
|
control
PGL = Primary Gill Lamella
SGL = Secondary Gill Lamella
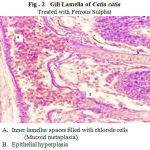 |
Figure 2: Gill Lamella of Catla catla. Click here to View figure |
Treated with Ferrous Sulphat
Inner lamellar spaces filled with chloride cells
(Mucoid metaplasia)
Epithelial hyperplasia
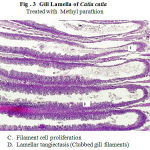 |
Figure 3: Gill Lamella of Catla catla. Click here to View figure |
Treated with Methyl parathion
Filament cell proliferation
Lamellar tangiectasis (Clubbed gill filaments)
References
- Anita Susan, T. Toxicity and effect of fenvalerate to the 3 major Indian carps, Labeo rohita, Catla catla, Cirrhinus mrigala (Ham.) by gas liquid chromatography. Res., 18(1): 57-59 (1994).
- Ashraf, M. Accumulations of heavy metals in kidney and heart tissues of Epinephelus microdon fish from the Arabian. Gulf. Environ. Monit. Asses., 101:311-316 (2005).
- Babu Velmurugan, Maryadoss Selvanayagam and ErhanUnlu. The effects of fenvalerate on different tissues of fresh water fish Cirrhinus mrigala. Environ. Sci.Hlth. 42(2):157-163 (2007).
- Bols, N.C., Brubacher,J.L., Ganassin R.C. and L.E.J. Lee. Ecotoxicology and innate immunity in fish. Comp. Immunol. 25(8):853-873 (2001).
- Caussy, D, Gochfeld, M. and E. Gurzau. Lesions from case studies of metals investigating exposure, bioavailability, and risk. Environ. Safety. 56:45-51 (2003).
- Ellar, L. L. Gill lesions in fresh water teleosts. In: Rivebelin. W.E. Migaki G. (Ed). The Pathology of Fishes, Univ. Wis. 305-330 (1971).
- Girija, M. Effect of heptachlor and dichlorovos on structure and function of gill tissues of a freshwater teleost, Tilapia mossambica. D., Thesis. S.V. Univ. Tirupathi. India (1987).
- Gupta, A. K. and V. K. Rajbanshi. Mercury poisoning. Architectural changes in the gill of Rasbora daniconius (Ham.). Environ. Biol. 16(1) 33-36 (1995).
- Hinton, D.E., Lauren, D.J., and C.S. Gian. Cellular composition and ultrastructure of hepatic neoplasm’s induced by diethyl notrosamine in Oryzias latipes. Environ. Res. 24:307-310 (1997).
- Javed, M. Studies on metals toxicity and physicochemistry of water in the stretch of river Ravi from Baloki headworks to Sidhnai barrage. I. J. Biol. Sci. 1:106-110 (2004).
- Jayantha Rao, K.Histopathology as a diagnostic tool in evaluation of toxicity. Toxicol. Selected Lectures and Methods. S. V. University, Tirupathi, India (1984).
- Jordao, C P and M G. Pereira. Assessment of water systems for contaminants from domestic and industrial sewages. Monit. Assess. 79:75-100 (2002).
- Kendall, M. W and J. E. Dale. Scanning and transmission electron microscope observations of rainbow trout gill. Fish Res. Bd. Can.36:1072-1079 (1989).
- Lohar, P.S. Comparative toxicity of four heavy metals in freshwater fishes. Aqua. Biol. 15(1/2):95-98 (2000).
- Mazhar Sultana and Dawood Sharief. Effect of heavy metals on the histopathology of gills and brain of Tilapia mossambica. Aqua. Biol. Vol. 19(1):165-168 (2004).
- Mosisch, T. and A. H. Arthington. Polycyclic aromatic hydrocarbon residues in the sediment of a dune lake as a result of power boating. Lakes & Reservoirs: Research and Management. 6:21-32 (2001).
- N. and S. Panigrahi. Histopathological abnormalities in the fish climbing perch, Anabas testudineus due to paper mill effluent. Environ. Ecol. 22: 24-25 (2004).
- Ramaiah, N. Bacterial diseases of Indian Major Carps and effects of chemotherapeutic drugs on the host and pathogen. M.F.Sc., Thesis. Coll. Fish., Mangalore (1982).
- Ramalingam K. and P. Murabai. A Time course test on LC50 determination of chlorine toxicity and its sub-lethal effects on tissue histology of Oreochromis mossambicus. Aquaculture, 3(2):125- 130 (2002).
- Ramamurthy, K. Impact of heptachlor on hematological, histological and selected biochemical parameters to the freshwater edible fish Channa punctatus. D. Thesis. S.V. University, Tirupathi, India (1988).
- Ramanakumari,C.Y. Toxicity and effect of chlorpyrifos on Indian major carp Labeo rohita. Phil., Dessertation. Nagarjuna University, Guntur, India (1999).
- Rameshkumar, B., Vijayalakshimi, S. and C. Rajmanickam. Toxicity effect of Zinc sulphate on gill in the freshwater fish, Mystus vittatus (Block). Abst,No.67. National Symposium On Ecotoxicology, Annamalai Nagar (1988).
- Rani, J. Ambrose, T. and P. Venkatesan. Tolerance and histopathological lesions in gill and intestine of Cyprinus carpio exposed to tannery effluent. Sem. Healthy Environ. For the Next generation, Loyola College (2004).
- Roberts, R. J. and H. D. Rodger. The pathophysiology and systemic pathology of teleosts. In: Fish Pathology, W. B. Saunders, London. pp 55-132 (2001).
- Roy, P.K. and J.S Datta Munishi. Malathion induced structural and morphometric changes of gills of fresh water fish Cirrhinus mrigala. Environ. Biol. 12(1): 79-87 (1991).
- Singer, C and S. Zimmermann. Induction of heat shock proteins (hsp70) in the zebra mussel (Dreissena polymorpha) following exposure to platinum group metals (platinum, palladium and rhodium): Comparison with lead and cadmium exposures. Aquatic toxicology. 75:65-75 (2005).
- Srivatsava, V.M. S. and R.S. Mauriya. Effect of chromium stress on gill and intestine of Mystus vittatus. Ecobiol. 3 (1):69-71 (1991).
- Subramanian, M.A.,Toxicology:Principles and Methods. MJT Publishers.p202 (2004).
- Sujatha, L. B. Studies on the Physiology, Haematology and Histology in the Indian Major Carp, Catla catla (Ham.) As influenced by individual and synergistic toxic effects of a pesticide and two metallic compounds. Ph. D., Thesis. University of Madras, Madras (2006).
- Tilak,K.S.,Koteswara Rao, D.,and K.Veeraiah Effects of chloripyrifos on histopathology of the fish , Catla catla. Eco. Environ. Monit., 15(2):127-140(2001 a).
- Tilak, K. S., K. and V. Janardhana Reddy. A study on Nitrite-Nitrogen effect on Haemoglobin content of the fish Puntius sophore and Channa punctatus. Poll. Res., 20(2):179-181 (2001).
- Tilak, K. S., Veeraiah, K. and K. Yacob. Studies on histopathological changes observed in the gill, liver, and kidney of Ctenopharyngodon idella exposed to technical fenvalelrlate and EC 20 %. Res., 20(3):387-393 (2001b).
- Tilak, K.S. and K. Yacob. Toxicity and effect of fenvalerate on fish Ctenopharyngodon idella exposed to technical fenvalerate and EC 20%. Res. 20(3):387-393 (2002).
- Tilak, K.S., Veeraiah, K.and Koteswara Rao. Histopathological changes observed in the Gill, Liver, Brain and Kidney of the Indian Major Carp Cirrhinus mrigala exposed to Chlorphrifos. Res., 24(1):101-111 (2005).
- V. V. and P. Gowri. Antigen induced by histopathological changes in liver and kidney of Labeo rohita. J. Ecotoxol. Environ. Monit., 12(3):209-213 (2002).
- Veena K. B and C. K. Radhakrishnan. Heavy metal induced biochemical effects in an estuarine teleost. Indian J. Mar. Sci., 26:74-78 (1997).
- Veeraiah, K. Cypermethrin toxicity and its impact on histochemical and histological changes in the Indian major carp, Labeo rohita (Ham.). D., Thesis. Nagarjuna University. Guntur, India (2001).
- Vijayalakshmi, S. and K. S. Tilak. Effect of pesticides on the gill morphology of Labeo rohita. Ecotoxi. Environ. Monit., 6(1):59-64 (1996).
- Vijayalakshmi, S. and K. S. Tilak. Effect of pesticides on the gill morphology of Labeo rohita. Ecotoxi. Environ. Monit., 6(1):59-64 (1996).
- Wannee Jiraung Koorshkul E., Prayad Pokethitiyook. Histopathological effects of round up, a glyphosate herbicide, on Nile tilapia Oreochromis niloticus. Asia. 28: 121-127 (2002).







