Manuscript accepted on :
Published online on: 05-12-2015
Plagiarism Check: Yes
D. S. Samiulla*1, A. Naidu2 and M. Ramachandra1
1DMPK Department, Aurigene Discovery Technologies Limited, Electronic City Phase 2, Hosur Road, Bangalore - 560 100 (India).
2Department of Chemistry, JNTUH, Kukatpally, Hyderabad - 500 085 (India).
Abstract
Caspases play a major role in apoptosis, and are considered to be key targets for the development of cytoprotective drugs. Previous studies have shown that irreversible caspase inhibitors are effective at protecting against endotoxic shock and lipopolysaccharide (LPS)/D-galatosamine liver injury model. This study involves the efficacy evaluation of broad spectrum, irreversible caspase inhibitors in preclinical models of anti-apoptotic therapy. Among the compounds tested, compound 3D significantly improved survival of mice in LPS induces apoptosis and acute lung injury in mice upon oral administration. It also exhibited a dose dependent inhibition of LPS/D-gal induced liver damage with ED50 of 1.01 mg/kg po, based on alanine aminotransferase activities. Protection from liver damage by compound 3D correlated well with suppression of liver tissue caspase-3 activity and drug levels. These results support further development of compound 3D as a potential anti-apoptotic agent.
Keywords
Caspase-3; small-molecule; Apoptosis; Inhibition
Download this article as:| Copy the following to cite this article: Samiulla D. S, Naidu A, Ramachandra M. Investigation of Orally Administered Small Molecule Inhibitors of Caspase-3 in Murine Models of Apoptosis. Biomed Pharmacol J 2012;5(1) |
| Copy the following to cite this URL: Samiulla D. S, Naidu A, Ramachandra M. Investigation of Orally Administered Small Molecule Inhibitors of Caspase-3 in Murine Models of Apoptosis. Biomed Pharmacol J 2012;5(1). Available from: http://biomedpharmajournal.org/?p=2173 |
Introduction
Programmed cell death is a highly regulated process involving the systemic disassembly and death of cells [1]. A key enzyme implicated in the apoptotic pathway includes a family of cysteine proteases, identified as caspases, which act in a cascade fashion to activate downstream caspases responsible for cleavage of key cellular substrates required for normal cell homeostasis [2-4].
The enzymatic cascade leading to apoptosis can be triggered through an extrinsic and intrinsic pathway. The former is driven by the cytokines such as Fas or TNF-a. There is now large body of data that show that caspase-mediated apoptosis accounts for at least part of cell death associated with variety of disease, such as ischemic stroke [5], acute respiratory syndrome, neurodegenerative disorder [4] and several liver diseases including alcoholic hepatitis, transplantation, Wilson’s disease, and viral hepatitis [6-7]. Several reports demonstrated that inhibition of caspases protect the liver from apoptosis-associated liver injury in preclinical models. Protypical caspase inhibitors such as ZVAD-FMK were efficacious in many animal models, including a-Fas- and TNF – mediated liver injury [7-8]. According to literature report, the broad-spectrum caspase inhibitors were efficacious in preclinical models suggesting the potential use in the treatment of liver diseases [4]. This indicates caspase-3 an interesting therapeutic target and the search for caspase–3 inhibitors has been a constant attempt by many pharmaceutical companies. In this paper, we evaluated the effect of five NCEs (Chemistry and Preliminary biological results were discussed earlier [9-11]) encompass better oral pharmacokinetic along with reasonable potency in the LPS induced endotoxic shock model and one NCE (Compound 3D) was further profiled in LPS/D-Gal induced liver injury model.
Materials And Methods
Reagents and General analytical methods
Caspase-3 substrate N-Acetyl-Asp-Glu-Val-Asp-7-amido-4-methylcoumarin (A1086), LPS (L2630), D-gal (G1639), pepstatin A, aprotinin, leupeptin, phenylmethylsulfonyl fluoride and other general reagent of analytical grade were obtained from Sigma, St. Louis, MO. IDN6556 was synthesized at Aurigene.
Fluorescence measurements were done using VICTOR2V 96/384 multilabel plate reader (PerkinElmer Life Sciences, MA) at λex=360nm and λem=460nm top readout) in Corning black 96-well flat bottom plates. The enzyme activity was measured at 30ºC after 30 minutes. LC-MS/MS was performed in Multiple Reaction Monitoring mode using Applied Biosystems API 3200 coupled to Agilent Technologies 1100 series HPLC on a reverse phase column (Zorbax Eclipse XDB C18, 50 x 4.6 mm, 5 µm).
Compound selection and screening
Biological screening of compounds as well as their chemical synthesis was based on a focused diversity approach belonging to four different series (indole fluromethylketone, indole difluro & tetrafluro phenoxymethylketone and oxalamide); which has been discussed in detail earlier [9-11] and summarized in Table 1 (potency as well as DMPK profile of the selected compounds from the above series).
Animals
All experimental procedures used in this study were approved by the institutional animal ethical committee (IAEC) based on the Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA) guidelines. For all the animal experiments, 8-10 weeks old male NMRI/balb-c mice with a body weight range of 25-40g were used. Mice were used for the experiments after one-week acclimatization to standard laboratory conditions, which were fed with standard diet and water ad libitum.
Pharmacodynamic studies
Endotoxic shock in the mouse model
Protection from lethal apoptosis induced acute lung injury was evaluated in male NMRI mice (n = 10 per group) using intravenous dose of LPS (from E. Coli serotype 0111:B4, Sigma, St. Louis, MO) at 30mg/kg and survival was monitored for 48 hours (Kawasaki et al., 2000; Weber et al., 2009). Compounds were administered at 1mg/kg po repeatedly at 0, 3, 6 and 9 hours post LPS injection. Three mice from each group were euthanized with isoflurane at 24 hour; lung tissue was harvested and frozen in liquid nitrogen for caspase activity.
Mouse liver apoptosis model
Assessment of protection from LPS (from E. Coli serotype 0111:B4, Sigma, St. Louis, MO) /D-Gal (Sigma, St. Louis, MO) induced liver injury model was performed as described [12, 7-8]. Male Balb-C mice (n = 7 per group) pretreated (0.5 h before) with either vehicle or 3D at different dose levels (0.1 to 10 mg/kg po) and were administered LPS/D-Gal intraperitoneally (0.2mg/kg, 800mg/kg i.p respectively dissolved in saline). Mice were euthanized with isoflurane at 6 h post LPS/D-Gal injection; Plasma was harvested for measurement of alanine aminotransferase (ALT) and drug levels. Liver tissue was harvested and frozen in liquid nitrogen for caspase activity as well as drug estimation. Plasma ALT level was determined using a standard diagnostic kit (Sigma) and were normalized to control in order to express in-terms of percentage. ED50 was calculated using Prism 5.1 software.
 |
Table 1: Profile of selected compounds used in this study
|
Caspase activity in lung and liver extracts
Effect of NCEs on lung and liver tissue extract caspase-3 activity was determined as described in Hoglen et al., 2003; Hoglen et al., 2001 [7-8], by measuring the cleavage of the fluorescent substrate (AC-DEVD-AMC). Briefly, tissues were homogenized in ice-cold hypotonic buffer (10mM HEPES, pH 7.4; 42mM KCl;50mM MgCl2.6H2O) containing 1mM dithiothreitol (DTT), 0.5% (w/v) CHAPS, and a cocktail of protease inhibitors [100mM EGTA, 100mM EDTA, 1mg/ml pepstatin A, 10mg/ml aprotinin, 1mg/ml leupeptin, and 1mM phenylmethylsulfonyl fluoride (PMSF)] and centrifuged at 12,000g for 15 min at 4°C. Protein concentrations of the resulting supernatant suspension were determined Bradford’s method with bovine serum albumin as the standard. The substrate cleavage activity was measured under similar conditions as mentioned earlier [9].
Data fitting and statistical analysis
 |
Figure 1: Structure known caspase-3 inhibitor and compound 3D
|
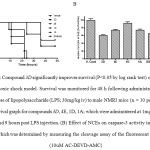 |
Figure 2: Compound 3D significantly improves survival (P<0.05 by log rank test) of mice in an endotoxic shock model. Survival was monitored for 48 h following administration of a lethal dose of lipopolysaccharide (LPS; 30mg/kg iv) to male NMRI mice (n = 10 per group). (A) Survival graph for compounds 3D, 4E, 1D, 1A; which were administered at 1mg/kg po at 0, 3, 6 and 9 hours post LPS injection. (B) Effect of NCEs on caspase-3 activity in the lung tissue, which was determined by measuring the cleavage assay of the fluorescent substrate (10uM AC-DEVD-AMC).
|
Curve fitting was performed with Prism 5.1 software (GraphPad, San Diego, CA), using built-in equation describing corresponding data models. All the other data were mentioned as mean ± standard deviation. Survival data is shown as Kaplan-Meier survival curves with statistical analysis by log-rank test. Difference in values were considered significant if P<0.05.
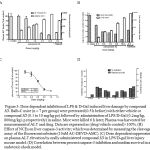 |
Figure 3: Dose dependent inhibition of LPS & D-Gal induced liver damage by compound 3D. Balb-C mice (n = 7 per group) were pretreated (0.5 h before) with either vehicle or compound 3D (0.1 to 10 mg/kg po) followed by administration of LPS/D-Gal (0.2mg/kg, 800mg/kg i.p respectively) in saline. Mice were killed 6 h later; Plasma was harvested for measurement of ALT and drug. Data are expressed as (drug/vehicle control)×100%. (B) Effect of NCEs on liver caspase-3 activity; which was determined by measuring the cleavage assay of the fluorescent substrate (10uM AC-DEVD-AMC). (C) Dose dependent suppression on plasma ALT elevation by orally administered compound 3D in LPS/D-gal liver injury mouse model. (D) Correlation between percent caspase-3 inhibition and median survival in an endotoxic shock model.
|
Results and Discussion
Excess apoptosis, which appears to be a detrimental process in a number of diseases including g acute respiratory syndrome and several liver disease such as Wilsons disease, viral and alcohol hepatitis [12-13, 6-8]. Caspase play an important role in different pathophysiological conditions and specific inhibition is expected to be therapeutically valuable [13-17]. Towards identifying inhibitors of caspases with desirable selectivity, ADME and efficacy profile; we screened a focused library of compounds and identified inhibitors from four chemical series that are distinct from other known small molecule caspase-3 inhibitors [9]. Table 1 shows profile of selected set of compounds from 3 series which exhibited better oral exposure were tested in rapid mice model of endotoxic shock along with the reported caspase inhibitor IDN6556 [7-8]. Male NRMI mice (n = 10 per group) were administered LPS (30mg/kg iv) and survival was monitored for 48 hours in presence and absence of test items. Compound 3D administered by repeat gavage (0, 3, 6 & 9 h post LPS) significantly improved survival (Figure 1A; P<0.05 log rank test) compare to other NCEs. It also suppresses the caspase-3 activity in lung tissue extract (figure 1B) similar to IDN6556 24 hours post injection. Compound 3D displayed survival advantage despite one log less potency when compare to IDN6556 in biochemical and cellular assay [9]. This may be due to better exposure in central circulation and low tissue partitioning property. Although permeability of all the tested compounds was low, modest cellular activity with compounds 3D may be because of its interaction with efflux pump [9]. Halogen (Cl) substitution on 5th position along with tetrafluorophenoxy substituent on right hand side of molecule (as in compound 3D) resulted in better oral exposure which in-turn showed advantages in lowering caspase-3 activity and significant improvement in the survival of mice in endotoxic shock model induced by intravenous injection of LPS [5].
The attractive profile of compound 3D prompted us to test further in a well-established mice model of liver injury [12, 18, 6-8]. Balb-C mice (n = 7 per grop) were administered LPS/D-Gal (0.2mg/kg, 800mg/kg i.p respectively) in saline and pretreated (0.5 h before) with either vehicle or compound 3D (0.1 to 10 mg/kg po). Analysis of plasma showed dose dependent inhibition of ALT levels including the increase in exposure in dose dependent manner (refer figure 2A & 3C). To investigate the effect of compound 3D on caspase activation, caspase-3 activity was measured using a fluorescent substrate in extracts of livers harvested 6 h after dosing. Caspase-3 activity was markedly inhibited upon compound 3D treatment in dose dependent manner (figure 2B) with ED50 value of 1.01 mg/kg (figure 2C). Overall we observed a dose dependent inhibition of LPS & D-Gal induced liver damage by compound 3D. The data suggest the therapeutic potential of this compound in indications where abnormally high amount of apoptosis occur. Compound 3D clearly demonstrated the efficacy by reducing the plasma ALT levels as well as caspase-3 activity in liver tissue extract with dose dependent increase in exposure levels both in plasma and liver. Because of its irreversible nature as well as selectivity over cysteine peotease, compound 3D could offer advantages specifically in better management of potential adverse effects considering that capsase activity is critically needed to maintain cellular homeostasis in normal cells. However, such potential advantages need to be confirmed by additional characterization of compound 3D in safety studies. In conclusion, this study supports the hypothesis that caspase inhibition may protect cells from abnormally large amounts of apoptosis seen in a number of disease states. Continual treatment with a caspase inhibitor may preserve the loss of critical numbers of cells responsible for the development of caspase-mediated cell death. These results support further development of compound 3D as a potential anti-apoptotic agent.
Acklowledgement
We gratefully acknowledge our collaborators Professor Saumitra Sengupta, Venkateshwar Rao G, Subhendu Mukherjee for providing the NCEs and molecular modeling support. The authors also would like to thank biochemistry, ADME and analytical departments for their assistance.
References
- Kerr, J.F.R., Wyllie, A.H., and Currie, A.R. “Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics.” Br. J. Cancer, 26: 239-257 (1972).
- Linton, S. D. “Caspase inhibitors: A pharmaceutical industry perspective.” Current Topics Med. Chem., 49: 7636-7645 (2005).
- Slee, E.A., Adrain, C. and Martin, S.J. “Serial killers: ordering caspase activation events in apoptosis.” Cell Death Differ., 6: 1067-1074 (1999).
- Nuttall, M.E., Lee, D., Ann, B., McLaughlin, N., and JErhardt, J.A. “Selective inhibitors of apoptotic caspases: implications for novel therapeutic strategies.” Drug Discov. Today, 6(2): 110-121 (2001).
- Kawasaki, M., Kuwano, K., Hagimoto, N., Matsuba, T., Kunitake, R., Tanaka, T., Maeyama, T. and Hara N. “Protection from lethal apoptosis in lipopolysaccharide-induced acute lung injury in mice by a caspase inhibitor.” Am. J. Pathol., 157(2): 597-603 (2000).
- Linton, S.D., Aja, T., Allegrini, P.R., Deckwerth, T.L., Diaz, J.L. and Hengerer, B. “Oxamyl dipeptide caspase inhibitors developed for the treatment of stroke.” Bioorg. Med. Chem. Lett., 14(10): 2685-91 (2004).
- Hoglen, N.C., Long-Shiuh, C., Fisher, C.D., Hirakawa, B.P., Groessl, T. and Contreras. P.C. “Characterization of IDN-6556 (3-{2-(2-tert-Butylphenylaminooxalyl)- amino]- ropionylamino}-4-oxo-5-(2,3,5,6- tetrafluoro-phenoxy)-pentanoic Acid): A Liver-Targeted Caspase Inhibitor.: J. Pharmaco. Exp. Ther., 309: 634–640 (2003).
- Hoglen, N.C., Hirakawa, B.P., Fisher, C.D., Weeks, S. and Srinivasan, A. et al., “Charecterization of the caspase inhibitor IDN-1965 in a model of apoptosis-associated liver injury.” J. Pharmaco. Exp. Ther., 297: 811-818 (2001).
- Samiulla, D.S., Naidu, A., Rao G.V., and Ramachandra, M. “Discovery of indole tetrafluorphenoxymehtlyketone, potent novel small molecule inhibitors of caspase-3.” Communicated to another journal & being considered; will be updated. 2012
- Sengupta, S., Rao, G.V. and Dubey P.K. “Synthesis and evaluation of novel oxalamide derivatives as caspase-3 inhibitors.” Indian J. Chem., 50B: 901-905 (2011).
- Sengupta, S., Rao, G.V. and Dubey, P.K., “Synthesis and Evaluation of Indole Aspartyl Ketones as Novel Caspase-3 Inhibitors.” Asian J. Chem., 23(12): 5517-5520 (2011).
- Yang, W., Guastella, J., Huang, J., Wang, J.C.Y. and Zhang, L. “MX1013: a dipeptide caspase inhibitor with potent in vivo antiapoptotic activity.” Br. J. Pharmacol., 140: 402-412 (2003).
- Okun, I., Malarchuk, S., Dubrovskaya, E., Khvat, A., and Tkachenko, S., et al., “Screening for caspase-3 inhibitors: A new class of potent small molecule inhibitors of caspase-3.” J. Biomol. Screening, 11(3): 277-285 (2006).
- Weber, P., Wang, P., Maddens, S., Wang, P.S.H., and Wu, R. et al., “VX-166: a novel potent small molecule caspase inhibitor as a potential therapy for sepsis.” Critical care. 13:R146 (2009).
- Scot, C.W., Sobotka-Briner, C., Wilkins, D.E., Jacobs, R.T., Folmer,J.J., et al., “Novel small molecule inhibitors of caspase-3 block cellular and biochemical features of apoptosis.” J. Pharmacol. Exp. Ther., 304: 433-440 (2003).
- Grobmyer, S.R., Armstrong, R.C., Nicholson, Gabay, S.C. and Arend, W.P. et al., “Peptidomimetic fluoromethylketone rescue mice from lethal endotoxic shock.” Mol. Med. 5: 585-594 (1999).
- Galle, P.R. “Apoptosis in liver disease.” J. Hepatol. 27: 405-412 (1997).
- Mignon A., Rouquet, N., Fabre, M., Martin, S. and Pages, J.C. et al., “LPS challenge in D-galactosamine-sensitized mice accounts for caspase-dependent fulminant hepatitis, not for septic shock.” Am. J. Respir. Crit. Care Med., 159: 1308-1315 (1999).
- Linton S.D., Aja, T., Armstrong, A., Bai, X., and Chen, L.S. et al., “First-in-class pan caspase inhibitor developed for the treatment of liver disease.” J. Med. Chem., 48: 6679-6782 (2005).







