Manuscript accepted on :December 16, 2010
Published online on: 25-11-2015
Plagiarism Check: Yes
Alok. Pareek*, P. E. Joseph and Daya S. Seth
School of Chemical Sciences, Department of Chemistry, ST. John’s College, Agra - 282 002 India.
Abstract
A convenient synthesis of substituted thiosemicarbazides & substituted thiosemicarbazones containing different functional groups have been synthesized by two different ways one is conventional heating method and other is microwave irradiation (MWI) method “Green Chemistry” .substituted thiosemicarbazides have been obtained by N(R)-malon anilic acid hydrazides (1a,1b) with substituted phenyl isothiocyanate in alcoholic medium and the substituted thiosemicarbazon-es have also been obtained by these two different techniques, derived from substituted aromatic aldehydes & ketone with substituted thiosemicarbazides (1a,1b) in ethanolic solution and few drops of glacial acetic acid was added. The results of the synthesized compounds in terms of yield, time, rapid reaction are compared. Some of the synthesized compounds(2a,2b,2d,2e,2h, 2i,3d,3f,4e,4f) were screened for their anti-bacterial activity against Staphylococcus aureus and Escherichia coli micro organisms. The structures of the synthesized compounds were characteri-zed by their physical properties, elemental analysis, spectral studies viz: IR, 1H NMR.
Keywords
Thiosemicarbazides; Thiosemicarbazones; Antibacterial Screening; Spectral Analysis
Download this article as:| Copy the following to cite this article: Pareek A, Joseph P. E, Seth D. S. Synthesis. Spectral Evaluation and Potential Antimicrobial Screening of some Substituted Thiosemicarbazides and Substituted Thiosemicarbazones Under Microwave Irradiation and Classical Heating. Biomed Pharmacol J 2010;3(2) |
| Copy the following to cite this URL: Pareek A, Joseph P. E, Seth D. S. Synthesis. Spectral Evaluation and Potential Antimicrobial Screening of some Substituted Thiosemicarbazides and Substituted Thiosemicarbazones Under Microwave Irradiation and Classical Heating. Biomed Pharmacol J 2010;3(2). Available from: http://biomedpharmajournal.org/?p=1606 |
Introduction
The chemistry and pharmacology of thio-semicarbazide, thiosemicarbazone have been of great interest to medicinal chemists for their wide range of biological activity1, Thiosemicarbazide and their derivatives are simple sulphur containing compound and possessing N-C-S group. In the recent year’s chemistry of thiosemi-carbazides and thiosemicarbazones have received much attention due to their use as intermediates for the synthesis of some heterocyclic systems.
Thiosemicarbazide and it’s derivatives have been reported to possess anti-bacterial2, anti-tubercular3, antifungal4, hypotensive5, herbicidal and growth regulating6, hypoglyceamic7 activity.
Thiosemicarbazone is a important class of heterocyclic chemistry and have shown unique spectrum as analytical, structural and biological activities. Substituted thiosemicarbazones have been found to possess antiviral8, antitumor9, anti-bacterial10, anti-tubercular11 activity .
Microwave assisted reactions attracted substantial attention in recent years, because of the simplicity in operation, milder reaction condi-tions, increasing reaction rates and formation of cleaner products. In particular microwave assisted “Green Chemistry” reactions12 have gained more popularity as they compared to conventional heating method. The present study is devoted to synthesize some substituted thiosemicarbazide & thiosemicarbazone derivatives by the both technique. In this context in continuation of our previous work13, IR, 1H NMR characterization and their antibacterial screening of some synthesized compounds have been reported. By various workers several substituted thiosemicarbazides & thiosemicarbazones have also been prepared in our laboratory14-18.
Experimental
Material and Methods
All the chemicals required for the present study were obtained from Sigma-Aldrich Com-pany Germany. Melting points were determined by open capillary tube method and using electro thermal apparatus were uncorrected. TLC was run on silica-gel-coated AI Plates using 10% (benzene/methanol). The IR spectra of the compounds were recorded on Perkin-Elmer spectrum RX-1 FT-IR spectrophotometer by using Kbr pellet technique and 1H-NMR of the synthesized compounds was recorded on Advanced Bruker DRX-300 spectrometer, DMSO was used as solvents, chemical shifts are given in (ppm) and protons signals are indicated as: s = singlet, d = doublet, t = triplet, m = multiplet. The physical and analytical properties of compounds are furnished in the Table-1 and spectral analysis are in the Table-2, antibacterial screening are recorded in the Table-3. The microwave irradia-tions for the synthesis of compounds were carried out in an IFB domestic microwave oven.
Synthesis of N(R)-phenyl malonamic acid hydrazide (a1-j1)
To the substituted aniline (0.025 mole), freshly distilled diethyl malonate (0.05 mole) was added with few drops of catalyst DMF, refluxed the reaction mixture for 45-minutes, add (20 ml) of ethanol to it, concentrated the reaction mixture over the boiling water-bath, add ethyl alcohol (20ml) with hydrazine hydrate 99%, the obtained solid part was purified by recrystallization from absolute ethanol.
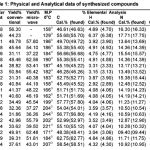 |
Table 1
|
General method A (heating) for the synthesis of substituted thiosemicarbazide (2a-2j)
The substituted phenyl anilic acid hydrazide (a1-j1; 0.001 mole), stirred solution of 4-fluoro phenyl isothiocyanate (0.001mole) in 20 ml of ethanol , the reaction mixture was refluxed for 3-hours, cooling, filtered, obtained solid part was recrystallized from ethyl alcohol 99%.
General method B (microwave irradiation) for the synthesis of substituted thiosemicarbazide (2a-2j)
To the (a1-j1; 0.001mole) and stirred solution of substituted phenyl isothiocyanate ( RI ; 0.001 mole), in (15 ml) absolute ethanol, were irradiated in microwave oven for 2-5 minutes. The obtained solid part was purified by recrystallization from hot absolute ethanol.
 |
Table 2
|
General method C (heating) for the synthesis of substituted thiosemicarbazone (3a-3f,4a- 4f)
A mixture of (2a,2b; 0.001 mole), substituted aldehydes and ketone (0.001 mole)in (20 ml) of absolute ethanol with few drops of glacial acetic acid, reaction mixture was refluxed for 3-hours, the solid part was obtained during refluxing period, cooling, filtered, it was purified by recrystallization from absolute ethanol.
General method D (microwave irradiation) for the synthesis of substituted thiosemicarbazone (3a-3f, 4a- 4f)
To the substituted thiosemicarbazide (2a, 2b; 0.001 mole) and stirred solution of substituted aldehydes and ketone in (15 ml) of absolute ethanol with 4 – 5 drops of glacial acetic acid as a
catalyst, reaction mixture was irradiated in microwave oven for 2-5 minutes, the obtained solid was purified by recrystallization from hot ethanol 99%.
Table 3: Antibacterial activity data of synthesized compounds
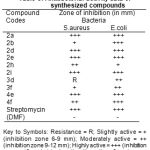 |
Table 3:
|
Key to Symbols: Resistance = R; Slightly active = + (inhibition zone 6-9 mm); Moderately active = ++ (inhibition zone 9-12 mm); Highly active = +++ (inhibition zone > 12 mm); (-) = inactive (Less than 6 mm).
Antibacterial Activity
The newly synthesized compounds were screened for their antibacterial activity against Staphylococcus aureus and Escherichia coli bacterial strains by the filter paper disc diffusion method19-20 was followed by using special Hi-Media sterile disc code 067. Control experiment was carried out using Streptomycin as a known standard antibacteria drug for comparison with the results at a concentration of 25 μg/ml. Screening was carried out in DMF solution. The bacteria were subcultured on nutrient agar medium and petridishes were incubated at 37OC for 24hrs. The results of the activity are given in Table-3.
Results and Discussion
The IR spectra (in Kbr) of synthesized compounds have been recorded in the frequency region 4000-500 cm-1 and 1H NMR Spectral data are recorded in the Table-2.
The IR spectra of the of substituted thiosemi-carbazide (2a) showed absorption frequencies (in cm-1) at 3441.9 (-NH), 3022.0(-CH), 1671.8 (CO NH), 1518.5 (C=O), 1429.3(-CH2 group), 1334.5 (C=S),1216.2(N-N), 671.0(mono substitution ring) .These infrared spectral analysis results Indicated the absorption spectrum was in agreement with the assigned structure of compound 2a, (2b,2d,2e) and other compounds 2c, 2f-2j. The IR spectra of compound (3e) and (4d) in Kbr showed absorption frequencies in (cm-1) at 3426.2 and 3445.5(-NH), 3022.2 and 3022.0(-CH), 2358.9 and 2358.0 (CH=C), 1676.4 and 1672.0(CONH), 1520.9 and 1518.4(C=O), 1338.1 and 1336.6 (C=S), 1216.5 and 1216.6(N-N), 671.9 and 671.9 (mono substitution ring). These results indicated the absorption spectrum was in agreement with the assigned structure of compound 3e, 4d and other compounds 3a-3d, 3f, 4a-4c, 4e-4f.
The 1H NMR spectra of compound 2a showed singlet at 3.358(CH2),7.170(NH),9.8 58(CONH), 10.264(C=S) and 1H NMR spectra of compound 2b showed doublet at : 3.3 36(-CH2), singlet at 7.171(-NH), 9.860(-CONH), 10.332(-C=S). These results are confirming the structure of compounds 2a, 2b and other newly synthesized compounds.
The results of antimicrobial screening indicates the title compounds showed moderate to strong activity against these two micro organisms.
Acknowledgements
The author thanks to Head, Central Drug Research Institute (CDRI), Lucknow for spectral data (IR, 1H-NMR) and Head, Department of Botany, Raja Balwant Singh College, Agra for valuable support in antimicrobial activity.
Referenc
- G.Mazzone, F.Bonia, R.A.Reina and G.Blan-dino, Farmaco Ed.Sci., 36: 181(1981).
- H.V.Patel and P.S.Fernandes,J.India.Chem. Soc., 67: 401-403(1990).
- Y.Aoki (Inst. Infectious Diseases, Tokyo) Japan, J.Bacterial, 9: 433-38(1986).
- A.Hameed Abou Shadi, Hany M.Safwat, Sonia, T.Hassib, M.Hussein and E.Salama, Egypt.J.Pharm.Sci., 24(1- 4), 159-68(1983).
- H.A.Schvoeder, F.M.Menhard and H.M.Perry Jr.,(Washington Univ., St. Louis,M.O.I)J.Lab. Chin.Med., 45: 431-440(1955).
- P.Inova and G.Vasilev (M.Popov inst.plant phylsiol., 113 Sofia,Bulg) Dokl Bolg. Akad. Nauk, 42(10),55-58(1989).
- Farbwerke Hoechest, G.Belg, 623, 263, Nov. 18(1963), Ger.Appl., May 12, 16pp.(1962).
- W.H.Wagner and E.Winkelmann, Arzeimfo-rsch, 22, 1713(1972); R.Protvinsky, Antibiot. Chemothes.,(Basal), 17: 101(1981).
- H.G.Petering, H.H.Buskirk and G.E.Under wood., Cancer Res., 64: 367(1963).
- P.Malatesta,G.P.Accinelli,G.Querlia., Ann.Chem., Rome, 149: 397(1959).
- R.Behnisch, F.Mietzsch and H.Schimdt, Amer .Rev.Tuberc., 61: 1-7(1950).
- A.V.Rao, A.Naqvi, Mohd. Shahnawaz, Daya S.Seth and P.E.Joseph, BioMed.& Pharm.J., 2(1): 185-188(2009).
- Alok K.Pareek, P.E.Joseph and Daya S.Seth, Orient.J.Chem., 26(1): 207-210(2010).
- R.K.Jain, Ibid, Agra Univ., Agra(1978).
- Mamta Agrawal, Ibid, Agra Univ., Agra(1980).
- Arun Kumar, Ibid, Agra Univ., Agra(1981).
- S.Bhatnager, Ph.D.Thesis, Agra Univ., Agra (1990).
- A.Chaudhary, G.Saxena, Shah.N.Khan, A. Naqvi and Daya S.Seth, Orient.J.Chem., 23 (3): 1089-1092(2007).
- R.Cruickshank, J.P.Duguid, B.P.Marion and R.H.A, Medicinal Microbiology, 12th Edn., 2: 196-202(1975).
- L.J.Bradshow Ed., A Text book of Microbio-logy, W.P.Sounders Co., Philadelphia, New York (1979).







