Manuscript accepted on :April 04, 2010
Published online on: 24-11-2015
Plagiarism Check: Yes
Alok K. Pareek*, P. E. Joseph and Daya S. Seth
School of Chemical Sciences, Department of Chemistry, ST. John’s College, Agra - 282 002 India.
Abstract
The present study deals with the synthesis and characteristics of some new substituted acid hydrazones synthesized by the condensation of R- malon anilic acid hydrazides (1a, 1b) with suitable substituted aldehydes and ketone in alcoholic medium. The synthesis was carried out by two different methodologies (classical heating and microwave irradiation) and compare these method of the synthesis in terms of yield, time etc. in this review showing the superiority of “Green technique” by microwave method over the conventional method. The newly synthesized compounds (1a,1b,2d,2e,2f,2h,3b,3c,3h,3j ) have been tested for their antibacterial activity against gram positive bacteria S. aureus and gram negative bacteria E. coli. The newly synthesized compounds were characterized by their physical properties, elemental analysis and spectral studies viz: IR, 1H NMR.
Keywords
Synthesis; Acid Hydrazones; Spectral studies; Anti-bacterial activity; Comparison
Download this article as:| Copy the following to cite this article: Pareek A. K, Joseph P. E, Seth D. S. Synthesis, Characterization and Pharmacological Screening of some Acid Hydrazones Under Classical Heating and Microwave Irradiation: A Comparative Study. Biomed Pharmacol J 2010;3(2) |
| Copy the following to cite this URL: Pareek A. K, Joseph P. E, Seth D. S. Synthesis, Characterization and Pharmacological Screening of some Acid Hydrazones Under Classical Heating and Microwave Irradiation: A Comparative Study. Biomed Pharmacol J 2010;3(2). Available from: http://biomedpharmajournal.org/?p=1577 |
Introduction
Acid Hydrazones possessing an azometine -NHN=CH- proton constitute an important class of compounds for new drug development therefore, many researchers have synthesized these compounds as target structures and evaluate their biological activities. Acid hydrazides have frequently been investigated for testing their potentiality as tuberculostats1-3, various hydrazides and their condensation products have displayed diverse range of biological properties such as bacteriocidal4, antifungal5, anticonvul-sant 6-8, anti-helmintic9, anti-tumor10-11, anti-leprotic12, anti-malerial13, anti-cancer14-15, anti-HIV 16, vasodilator17 activities. Some hydrazones and their derivatives have been reported to possess bactericidal18 property. Hydrazones have also been found to possess anti-bacterial19-21, anti-fungal22, antiviral23, insecticidal24 activities. Some substituted hydrazones have been represents acetyl choline strease inhibitory activity25, acid hydrazones have proved their application in analytical chemistry, they have been used as chelating agents for the quantitative estimation of transition metal ions26, hydrazones have also gained commercial significance as charge transporting agents in electro photographic photo receptors27. In the last few years Microwave induced organic reaction enhancement (MORE), chemistry has gained popularity as a non-conventional technique for rapid organic synthesis28 and many researchers have described accelerated organic reactions, many papers have appeared for proving the synthetic utility of MORE chemistry in comparison of routine conventional organic synthesis.It can be termed as e-chemistry because it is easy, effective, economical and eco-friendly and is believed to be a step towards “Green Chemistry”.
In view of the above and In continuation of our previous work29-30 in the present study we have synthesized a series of substituted acid hydrazones by classical heating and compare with microwave irradiation (MWI) method, we propose to present a very simple, fast and eco-friendly method for the synthesis of substituted acid hydrazones by the reaction of substituted acid hydrazides with suitable substituted aldehydes and ketone. The synthesized compounds were tested for their antibacterial activity against gram positive bacteria S.aureus and gram negative bacteria E.coli.
Experimental
Material and Methods
The melting points were determined in open capillary tubes and are uncorrected.
 |
Table 1
|
All the used chemicals were of analytical AR grade. The purity of the synthesized compounds were checked on silica-gel-coated AI Plates (Merck). The structures of the synthesized compounds were determined by elemental analysis, IR and 1H NMR spectral data. IR spectra(in Kbr) are recorded on a Perkin-Elmer spectrum RX-1 FT-IR spectrophotometer at ST. John’s College – Agra, 1H NMR Spectra was measured on Advanced Bruker DRX-300 using solution in DMSO d6 . Chemical shifts are given in (ppm) and protons signals are indicated as: s=singlet, d=doublet, t=triplet, m=multiplet. Micro-wave irradiations were carried out in an IFB domestic microwave oven.
 |
Scheme 1
|
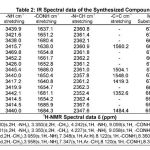 |
Table 2
|
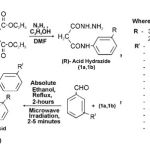
General method for the synthesis of N(R) phenyl malon anilic acid hydrazide (1a, 1b) .
A mixture of (3-CI-4-OCH3, 2-OCH3-5-CH3; 0.025 mole) and freshly distilled diethyl malonate(0.05 mole) in presence of dimethyl formamide as a catalyst was refluxed for about 45 minutes, contents were cooled, ethanol (20 ml) was added, concentrated over boiling water-bath then treated with ethanol (20 ml) and hydrazine hydrate 99%. The mixture was set a side and the solid product was separated out, purified by recrystallization from hot ethanol, it was identified as N(R) phenyl malon anilic acid hydrazide (1a,1b), R= 3-chloro-4-methoxy, 2-methoxy-5-methyl aniline.
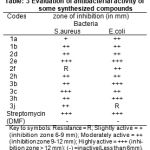 |
Table 3
|
General method A (Heating) for the synthesis of substituted acid hydrazones (2a-2m, 3a-3m)
The stirred solution of (1a,1b ; 0.001 mole) and (0.001 mole) of substituted aldehydes (as benzaldehydes) and ketone dissolved in absolute ethanol (10 ml) and was refluxed for about 2-hours, the solid part was obtained on cooling and then filtered to give the corresponding substituted acid hydrazones which was recrystallized from hot ethanol 99%.
General method B (Microwave irradiation) for the synthesis of substituted acid hydrazones (2a-2m, 3a-3m) .
The substituted acid hydrazide (1a,1b; 0.001 mole) and (0.001 mole) of substituted aldehydes (as benzaldehydes) and ketone in (15 ml) of ethanol were irradiated in microwave for 2-5 minutes, the solid product was obtained, the resulting solid was purified by recrystallization from absolute alcohol several times.
Antibacterial activity
For bacteria nutrient agar medium was used Staphylococcus aureus and Escherichia coli were selected for the screening. The antibacterial activity was compared with known standard antibacteria drug streptomycin and zone of inhibition (in mm) of antibacterial activity was determined by using filter paper disc diffusion method31-32 was followed by using special Hi-Media sterile disc. All of the mentioned compounds for screening was evaluated at 25µg ml-1 concentration. The synthesized compounds were tested as a suspension in DMF. The petridishes were incubated at 37OC for 24hrs.
Results and Discussion
The IR (in Kbr) spectrum of the synthesized compounds have been recorded in the frequency region 4000-500 cm-1. The spectral data of IR and 1H NMR are recorded in the Table-2.
The infrared spectra of N(3-Cl-4-OCH3) phenyl malonamic acid hydrazone of furfur-aldehyde2k shows -NH stretching vibrations at 3440.0 cm -1, absorption bands at 1650.4 cm-1 represents -CONH stretching vibrations, absor-ption at 2357.8 cm-1 reveals aromatic -N=CH, absorption at 1548.0 cm-1 indicating ring stretching C=C, mono substitution are present at 672.9 cm-1 . The IR spectrum of N(2-methoxy-5-methyl) phenyl malonamic acid hydrazide of 4-hydroxy3a are representing -NH stretching vibrations at 3417.2 cm-1, while absorption at 1640.8 cm-1 indicates the -CONH, stretching vibrations at 2361.2 cm-1 shows aromatic -N=CH, absorption at 1419.3 cm-1 reveals -C=C, mono substitution are seen at 670.0 cm-1. These infrared spectral observations are indicating the absorption spectrum was in agreement with the assigned structure of compounds 2k,3a and (2a-2j), (3b-3f) and other compounds 2l-2m and 3g-3m. The 1H NMR spectra shows singlet at 2.500, 2.501(NH2), and 3.350(CH2), doublet at 3.350, 3.339,3.339(CH2), singlet at :4.242,4.227, 3.959, 3.958(-NH), singlet at:9.059,9.058,8.122,
8.120(-CONH). These observations are confir-ming the structures of compounds 1a,1b,2h,3h and other compounds.
The results of antimicrobial activity indicates the compounds showed moderate to strong activity against the gram positive and gram negative bacterial strains. All title antibacterial screening results are shown in the Table-3.
Acknowledgements
The author is thankful to The Head, Central Drug Research Institute (CDRI), Lucknow for spectral data (1H NMR) and Head, Department of Botany, R.B.S College, Agra for antimicrobial screening.
References
- S.K.Agrawal,R.Chandra, R.Gupta, D.R.Tutlani ,J.Inst.Chem.,(India),59(5)225(1987);C.A.Vol.108,164594w(1988).
- A.A.Martynovskii, B.A.Samura and Cowerkers , Med.Inst.Zaporrze, USSR, Khim. Farm.Zh., 24(7), 31-2(1990), C.A.113: 164948t(1990).
- Strokin, V.Yu, I.A.Karasovskii and Cowerkers, (Bashk.Med.Inst., UFA,USSR), Khim.Farm.Zh, 24(7)45-8 (1990),C.A.114:172e(1991).
- T.S.Gardner, E.Wenis and J.Lee, J.Org.Chem ., 26: 1514(1961).
- A.Omar, M.E.Mohsen, A.M.Farghaly, A.A.B. Hazzai, N.H.Eshba, (Fac. Pharm.Univ.Alexan dria,Egypt). Pharmazie (1980); C.A.95: 25382 a(1981).
- S.Rollas, S.G.Kucukguzel, Molecules,12:1910 -1939(2007).
- S.Rollas, N.Gulerman, H.Erdeniz, Farmaco., 57: 17(2002).
- K.B.Kaymakcioglu, E.E.Oruc, S.Unsalan, F. Kandemirli, N.Shvets, S.Rollas, D.Anatholy; Eur.J.Med.Chem ., 41: 1253-1261(2006).
- R.Kalsi, M.Shrimali, T.N.Bhalla, J.P.Barthwal; Indian J.Pharm.Sci., 41: 353-359(2006).
- J.Ragavendran, D.Sriram, S.Patel, I.Reddy, N.Bharathwajan, J.Stables, P.Yogeeswari, Eur .J.Med.Chem., 42: 146-151(2007).
- U.Salgin-Goksen,N.Gokhan-Kelekei,O.Goktas ,Y.Koysal, E.Kilic, S.Isic, G.Aktay, M.Ozalp, Bioorg.Med.Chem., 15: 5738-5751(2007).
- A.Masunari, L.C.Tavares, Bioorg.Med.Chem., 15 : 4229-4236 (2007).
- A.Bijev, Lett. Drug.Des. Discov., 3: 506-512 (2006).
- T.Scior, S.J.Garces-Eisele, Curr.Med.Chem., 13: 2205-2219(2006).
- Y.Janin, BioOrg. Med. Chem., 15: 2479-2513 (2007).
- L.Savini, l.Chiasserini, V.Travagli, C.Pellerano







