Manuscript accepted on :December 10, 2010
Published online on: 24-11-2015
Plagiarism Check: Yes
N. N. Nwobodo¹* and P. O. Okonkwo²
¹Department of Pharmacology and Therapeutics, College of Medicine, Enugu State University of Science and Technology, Enugu Nigeria.
²Department of Pharmacology and Therapeutics, College of Medicine, University of Nigeria, Enugu Nigeria.
Corresponding Author E-Mail:nnwobodo@yahoo.com
Abstract
Malaria patients (n=60) confirmed by thick blood film and immunological tests were equally categorized into test and control groups in a double blind randomized controlled trial. The test group received chloroquine and simvastatin while the control received chloroquine alone. Patients were followed up on days 0, 3, 7, 14 and 28 post-treatment. Assessment of parasitological response was carried out in line with WHO criteria. Results revealed that low (RI), moderate (RII) and high (RIII) level parasitological resistance were significantly (P<0.05) reduced in the test group relative to control. Similarly, the late parasitological failure (LPF) was significantly (P<0.05) reduced in the test relative to the control group in the study.In conclusion, the significant decline in total parasitological resistance noted in the test group relative to control could be rightly attributed to the modulating influence of simvastatin.
Keywords
Chloroquine; parasitological resistance; simvastatin; uncomplicated malaria
Download this article as:| Copy the following to cite this article: Nwobodo N. N, Okonkwo P. O. Simvastatin Reverses Parasitological Resistance in Malaria Patients Treated with Chloroquine. Biomed Pharmacol J 2010;3(2) |
| Copy the following to cite this URL: Nwobodo N. N, Okonkwo P. O. Simvastatin Reverses Parasitological Resistance in Malaria Patients Treated with Chloroquine. Biomed Pharmacol J 2010;3(2). Available from: http://biomedpharmajournal.org/?p=1564 |
Introduction
Chloroquine resistant malaria has been a major public health threat globally, particularly in sub-Saharan Africa. This has necessitated the replacement of chloroquine as the first-line therapy for uncomplicated malaria. Indeed, resistance has intensified to the point where chloroquine cannot, by any reasonable definition, be considered to have any clinical efficacy.
A number of hypotheses have been proposed to explain the Plasmodium falciparum resistance to chloroquine. It has been shown from many years of investigation that chloroquine resistant parasites generally accumulate less chloroquine than their sensitive counterparts1. This had led to the assumptions that chloroquine resistance was a simple phenomenon, although it may have a polygenic basis and that the resistance process was related in some way to altered drug transport and intra-parasitic accumulation. Evidence put forward in support of the efflux hypothesis was based upon differences in the absolute rates of drug efflux in resistant versus sensitize Plasmodium falciparum2,3,
Patients And Methods
Sixty subjects, with clinically characterized frank malaria confirmed by thick blood film and immunological tests were enrolled in a double blind randomized controlled study. The subjects were selected from patients attending 8 primary health facilities within Asu Nkanu Local Health Authority in Nkanu East Local Government Council of Enugu State, Nigeria. Informed consent was obtained by formal written documentation after adequate explanation of the purpose of study, type of treatment to be administered and clarification of any likely adverse effects or complication that may arise. Ethical clearance certification was obtained from health research ethics committee, University of Nigeria Teaching Hospital and ethics review committee of the Enugu State Health Board. Medical history was obtained and clinical examination carried out to ascertain each subject’s physical condition and exclude the presence of confounding ailments. Randomization of subjects into control and test groups were carried out using a table of random numbers which was statistically generated. The investigator, microscopist, field supervisor and assistants had no prior knowledge of the treatment group to which each subject was assigned. The test group received chloroquine (administered orally as 10mg/kg D0, followed by 5mg/kg 6 hours later, then 5mg/kg daily for the next two days, D1 and D2) and simvastatin (0.6mg/kg/d) given once in the evening for 3 consecutive days; while the control group received chloroquine alone. Baseline liver function tests were carried out before commencement of therapy and periodically thereafter. Treatment was discontinued if elevation of serum transaminase activity, up to three times the normal level occurred. Patients were followed up on days 0, 3, 7, 14 and 28.
Assessment of Parasitological Response
Parasitological response was classified as low to high parasitological resistance (RI, RII, RIII).
Resistance III (RIII)
Parasitemia on day 3, D3 higher or 25% of parasitemia on D0.
Resistance II (RII)
Parasitemia on day 3, D3 ≤ 25% of parasitemia on D0; but positive parasitemia between D4 and D7.
Resistance I (RI)
A negative blood smear on day 3, D3 and a positive blood smear on any day between D7 and D14.
Late Parasitological Failure (LPF)
Presence of parasitemia on D28 and axillary temperature <5oC without previously meeting any of the criteria of early treatment failure or late treatment failure.
Data obtained were analyzed using Graphpad Prism statistical software and presented in tabular and graphical forms. Statistical test of significance between test and control groups ascertained using two-tailed Student t-test assuming equal variance at 95% confidence interval, P<0.05 considered significant.
Results
Table I and Figure 1, depicted parasitological resistance in both test and control malaria patients. Low level parasitological resistance (RI) in the test subjects was given as 13.2% as compared to 21.5% in the control. Similarly, mid-level parasitological resistance of 11.4% in the test subjects contrasted with value of 24.4% in the control. Again, high level parasitological resistance of 4.7% in the test relative to 18.3% in the control subjects. It was also shown that the late parasitological failure (LPF) of 2.7% in the test differed from 6.3% given in the control. A statistically significant difference (P<0.05) was reported between test and control subjects in all the assessed parameters, as shown by two-tailed Student t-test at degree of freedom df=28.
Table 1: Parasitological Resistance In Both Test And Control Malaria Patients
|
TREATMENT GROUPS MEAN (SEM)
|
RI (%) | RII (%) | RIII (%) | LPF (%) |
| TEST | 13.2(0.91) | 11.4(0.88) | 4.7(0.19) | 6.5(0.54) |
| CONTROL | 21.5(0.87) | 24.4(0.65) | 18.5(0.68) | 2.7(0.13) |
| P-Value | P<0.05 | P<0.05 | P<0.05 | P<0.0 |
*RI: Resistance I
*RII: Resistance II
*RIII: Resistance III
*LPF: Late Parasitological Failure
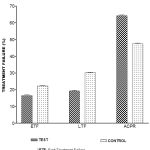 |
Figure 1:
|
Discussion
The incidence of low to high level parasitological resistance (RI + RII + RIII) reported in the present study varied from 29.3% in the test subjects to 64.4% in the control treated with chloroquine alone. The consideration of late parasitological failure (LPF) of 2.7% and 6.5% alongside gave a total parasitological resistance of 32% and 70.9% for the test and control subjects respectively. The results of a Malawian study revealed a total resistance of 79.7% (RI + RII + RIII)4. A similar study in Zambia reported that incidence of moderate to high level parasitological resistance (RII + RIII) was 50.5%5. This study reported high level parasitological resistance (RIII) of 4.7% and 18.5% for the test and control subjects respectively. A study suggested that when the level of RIII parasitological response exceeded 5-10% careful evaluation of the parasitological response should be conducted6. The outcome of this evaluation, if suggestive that the median duration of parasitological response was below 14 days and/or hematological recovery was not optimal, a change in first line treatment would be indicated. It therefore seemed safe to infer that repeat presentations within 14 days by malaria patients who have taken a full course of medication would mainly be due to resistant infections. The confirmation of diagnosis of malaria in patients re-presenting within 14 days of anti-malarial treatment was vital, to avoid confusion of treatment failures with other malaria-like conditions. Thus, a simple antigen-detection test as was employed in the present study was inevitable to carry out such confirmation. However, as circulating parasite antigen persisted for some time after an infection has been cured; confirmation of resistant cases with such tests were only reliable in cases presenting greater than 9 days post-treatment7. A group suggested that using economic modeling, a change from chloroquine to another anti-malarial drug was justified when the prevalence of RIII parasitological resistance to chloroquine was in the range 14 to 31%. The above analysis was based on assumptions that were no longer valid. Total parasitological resistance to chloroquine in vivo of 67% was reported in Colombia8. This comprised of low to high level of parasitological resistance of 47% and late parasitological failure of 20%. The concordance between in vivo and in vitro resistance was 33%. The above compared closely with the 67.3% reported for control subjects in this study. However, unlike the Colombian study in vitro drug resistance was not assessed in the present study. The late parasitological failure of 20% reported in the Colombian study differed significantly from the values reported in both test and control subjects in this study. High levels of parasitological resistance to chloroquine such as this have been reported in other malarial zones of the world9-11. It has been recommended by the World Health Organization that evaluation of sensitivity of Plasmodium falciparum to anti-malarials was better assessed by in vivo tests, since they reflected better the biological interaction with the drug and host’s response10.
Liverpool studies have confirmed the reduced levels of chloroquine accumulation and selective sensitivity enhancement by verapamil in highly chloroquine resistant isolates. The chloroquine dose-response in these isolates never returned to the levels seen in truly chloroquine susceptible isolates (with IC50 around 10nM). The IC50 obtained for resistant isolates with chloroquine plus verapamil were typically some three-fold higher than this. The chloroquine IC50 estimates indicated that the isolates were resistant, according to WHO statements12. Moreover, these concentrations are among those reported in previous studies of chloroquine Plasmodium falciparum resistance reversal13-15.
This could be interpreted as a possible evidence for the existence of more than one resistance phenotype in highly resistant parasites16. The analysis of data based on kinetic model indicated that initial rate of drug accumulation was faster and steady-state drug levels were achieved later in drug sensitive parasites than in resistant lines17.
The ability of the classic reverser of multi-drug resistance in cancer cells to increase chemosensitivity to chloroquine in resistant isolates of Plasmodium falciparum in vitro was by far the most compelling piece of evidence for an efflux-based mechanism. This sensitivity enhancement has been associated with a higher level of drug accumulation in resistant parasites compared with that of the sensitive ones, albeit at pharmacologically irrelevant drug concentration18. Simvastatin, a 3-HMG-CoA reductase inhibitor, has been shown to exhibit important immunomodulatory effects independent of lipid lowering19,20.
Conclusively, in view of the foregoing, the significant decline in the parasitological resistance noted in malaria patients treated with chloroquine and simvastatin relative to those treated with chloroquine alone could rightly be attributed to the modulating influence of 3-HMG-CoA reductase inhibitor simvastatin.
References
- Bray P.G. and Ward S.A. Malaria chemotherapy: resistance to quinoline containing drugs in Plasmodium falciparum. FEMS Microbiology Letters. 113: 1-8 (1998).
- Krogstad D.J., Gluzman I.Y., Kyle D.E., Oduola A.M.J., Martins S.K. and Schlesinger P.M. Efflux of chloroquine from Plasmodium falciparum: mechanism of chloroquine resistance. Science. 235: 1283-1285 (1987).
- Bray P.G., Howells R.E, Ritchie G.Y. and Ward S.A. Rapid chloroquine efflux phenotype in both chloroquine sensitive and chloroquine resistant Plasmodium falciparum. Biochemical Pharmacology. 44: 1317-1324 (1992).
- Bloland P.B., Kazembe P.N., Oloo A.J., Himonga B., Barat L.M. and Ruebush T.K. Chloroquine in Africa: critical assessment and recommendations for monitoring and evaluating chloroquine therapy efficacy in sub-Saharan Africa. Tropical Medicine and International Health. 3(7): 543-552 (1998).
- Barat L., Himonga B., Nkunika S. et al. A systematic approach to the development of national malaria treatment policy in Zambia. Tropical Medicine and International Health. 3: 535-542 (1998).
- Bloland P.B., Lackritz E.M., Kazembe P.N., Were J.B.O., Steketee R. and Campbell C.C. Beyond chloroquine: implication of drug resistance for evaluating malaria therapy efficacy and treatment policy in Africa. Journal of Infectious Diseases. 167: 932-937 (1993).
- Mharakurwa S. and Shiff C.J. Post-treatment sensitivity studies with the ParaSight®_F test for malaria diagnosis in Zimbabwe. Acta Tropica. 66: 61-67 (1997).
- Blair-Trujillo S., Lacharme-Lora L. and Carmona-Fonseca J. Resistance of Plasmodium falciparum to anti-malarial drugs in Zaragoza (Antioquia-Colombia). Inst. Oswaldo Cruz. 97(3): 401-406 (2002).
- Al Yaman F., Genton B., Mokela D., Narara A., Raiko A. and Alpers M.P. Resistance of Plasmodium falciparum malaria to amodiaquine, chloroquine and quinine in the Madang Province of Papua New Guinea. PNG Med. J. 39: 16-22 (1996).
- Ekvall H., Premji Z and Bjorkinen A. Chloroquine treatment for uncomplicated childhood malaria in an area with drug resistance: early treatment failures aggravate anemias. R. Soc. Trop. Med. Hyg. 92: 556-560 (1998).
- Di Perri G., Olliaro P., Narali S., Deganello R., Allegranzi B., Bonora S., Vento S. and Concia E. Response of uncomplicated falciparum malaria to oral chloroquine and quinine in Bumrah highlands. Trop. 70: 25-33 (1998).
- Bruce Chwatt L.J. Chemotherapy of Malaria. 2nd WHO, Geneva. pp. 211-233 (1986).
- Bitonti A.J. and McCann P.P. Desipramine and cyproheptadine for reversal of chloroquine resistance in Plasmodium falciparum. Lancet ii: 1282-1283 (1989).
- Basco L.K and Le Bras J. Reversal of chloroquine resistance with desipramine in isolates of Plasmodium falciparum from Central and West Africa. R. Soc. Trop. Med. Hyg. 84: 479-481 (1990).
- Kyle D.E., Oduola A.M.J., Martin S.K. and Milhous W.K. Plasmodium falciparum modulation by chloroquine, desethylchloroquine, quinine and quinidine in vitro. Trans. R. Soc. Trop. Med. Hyg. 84: 474-478 (1990).
- Ward S.A., Bray P.G., Munythin M. and Hawley S.R. Current views on mechanisms of resistance to quinoline-containing drugs in Plasmodium falciparum. Annals of Trop. Med. and Parasitol. 89(2): 121-124 (1995).
- Ginsburg H. and Stein W.D. Kinetic modeling of chloroquine uptake by malaria infected erythrocytes. Biochemical Pharmacology. 41: 1463-1470 (1991).
- Krogstad D.J., Gluzman I.Y., Kyle D.E., Oduola A.M.J., Martins S.K. and Schlesinger P.M. Efflux of chloroquine from Plasmodium falciparum: mechanism of chloroquine resistance. Science. 235: 1283-1285 (1987).
- Weitz Schimidt G., Welzenbach K., Brinkmann V. et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Med. 7: 687-692 (2001).
- Kwak B.R., Mulhaupt F., Myit S. and Mach F. Statins as a newly recognized type of immunodulator. Med. 6: 1399-1402 (2000).







