Manuscript accepted on :April 15, 2010
Published online on: 24-11-2015
Plagiarism Check: Yes
Deepak K. Ahirwar, Rahul Jain, Sangita Chourasia, R. C. Saxena and D. K. Jain
Pest Control and Ayurvedic Drug Laboratory S.S.L. Jain P.G. College Vidisha India.
Abstract
In this present study Cassia fistula (caesalpinaceae) fruits were extracted in 90% alcohol and water. The extract was vacuum evaporate to driness which yield 26.02% The extract was evaluated for hepato protective activity against CCI4 induced liver damage 50m/g 100mg/kg body weight. The biochemical parameter observed in the serum were Asparate transminase (AST), Alanine transaminase (AST), Alkaline phosphatase (AST), total protein and total lipid, Histopathologicla studies on the liver tissue were also performed. The extract exhibited dose dependent reduction in (AST), ALT and ALP and increase in total protein levels. Extracts thus Cassia fistula extract attenuates the hepatotoxic effect of CCI4.
Keywords
Cassia fistula; CCl4; Albino rats
Download this article as:| Copy the following to cite this article: Ahirwar D. K, Jain R, Chourasia S, Saxena R. C, Jain D. K. Hepatoprotective Activity of Cassia Fistula Alcohlic Extract Against CCl4, Induced Liver Damage in Albino Rats. Biomed Pharmacol J 2010;3(1) |
| Copy the following to cite this URL: Ahirwar D. K, Jain R, Chourasia S, Saxena R. C, Jain D. K. Hepatoprotective Activity of Cassia Fistula Alcohlic Extract Against CCl4, Induced Liver Damage in Albino Rats. Biomed Pharmacol J 2010;3(1). Available from: http://biomedpharmajournal.org/?p=1462 |
Introduction
Liver regulates various impor tant metabolic functions, hepatic damage is associated with distortion of these metabolic functions (Wolf 1999), liver disease are still a world wide health problem. Unfortunately conventional or synthetic drugs used in the treatment of liver disease are inadequate and some times can have serious side effects. This is one of the reason for many people in the world over including those in developed countries turning complimentary and alternative medicine (CAM).
Cassia fistula (Casealpinaceae) tree is one of the most widespread in the forest of India, usually in deciduous forest. The whole plant possesses medicinal properties useful in the treatment of skin disease, inflammatory diseases, rheumatism, anorexia and jaundice (Anonymous, 1992, Kirtikar and Baue 1991). A new bioactive flavon glycoside is reported by (Yadav and Verma, 2003).
However a detailed pharmacological screening of the Cassia fistula pod extracts have not been reported. The present study reports the heapato protective activity of Cassia fistula pod extracts.
Materials
Plant Material
The pods of Cassia fistula Linn (Caesalpinaceae) were collected from Vidisha, MP, India during the month of Sept. and identified by Dr. S.K. jain dept of botany. The pods were dried in shade for a week. Then powdered and extracted with 90% alcohol and water by sox let extraction (24 hrs) to yield the extract. The extracts were vacuum dried in a rotary vacuum evaporator and the extractive yield was 26.02%.
Inbred Wistar albino male rats (100- 120gm) were used for the evaluation of pharmacological activities. They were kept in colony.
Table 1: Protective effect of Cassia fistula fruits extracts on carbon tetrachloride induced different biochemical parameters in the serum of rats
| Group-I control with LP only | Group–II LP+ control | Group-III LP+CCI | Group-IV LP+CCI +CF | Group-V LP+CCI Silymerin | ||
| +CF50mg/kgb.w. | 100mg/kg b.w. | 4 | ||||
| +CF 200mg/kg 100mg/kg | ||||||
| b.w. control b.w. | ||||||
| Aspartate | 22.42±0.68 | 35.72±1.48 | 32.92±1.30 | 25.68±1.20 | 23.79±1.20* | 23.64±1.28 |
| Transaminase% | ||||||
| Alanine | 25.12±1.54 | 63.20±2.98 | 56.20±2.68 | 40.28±2.14 | 29.98±2.16* | 26.98±2.16 |
| Transaminase% | ||||||
| Alkaline | 69.84±3.82 | 122.32±3.42 | 120.40±3.40 | 85.30±3.40 | 78.40±3.52* | 75.06±3.14 |
| Phosphatase% | ||||||
| Total protein | 6.82±0.46 | 4.28±0.18 | 4.42±0.26 | 5.42±0.44 | 5.96±0.43* | 6.14±0.40 |
| (g/100mLserum) | ||||||
| Total lipid | 178.46±5.62 | 257.78±8.92 | 234.82±7.42 | 182.24±6.12 | 146.42±5.14* | 138.24±5.22 |
| (mg/100gcerum) | ||||||
| Tryglycerides(mg/100g | 8.18±0.62 | 14.12±1.62 | 14.02±1.38 | 12.96±1.16 | 9.24±0.55* | 9.02±0.52 |
| cerum) | ||||||
| Colesterol | 62.38±3.21 | 101.02±5.24 | 93.14±5.12 | 81.62±4.14 | 78.69±4.08* | 63.21±4.12 |
| (mg/100gcerum) | ||||||
| Phospholipid | 119.30±6.84 | 250.32±12.08 | 214.22±10.08 | 156.28±8.02 | 140.28±8.32** | 146.30±8.64 |
| (mg/100g cerum) | ||||||
* P<0.5 as compared to group – I
** P< 0.10 as compared to group – II
Value are mean ± SE from 6 animals of each group cages at 25± 2°C, relative humidity 45-55% under 12hrs light and dark cycles. All the animals were kept acclimatized for a week before use. They were fed with standard animal feed (Hindustan lever ltd) and water adlibitum. The test compound and the standard drugs were administered in the form of a suspension using 5% gum acacia as vehicle, to each group consisted of six animals.
All the pharmacological experimental protocols were perfor med according to the recommendation of the CPCSEA and institutional animals ethical committee.
Experimental bioassay methodologies
Rats were divided into six groups of six animals in each groups Group I (control) animals were administered a single daily dose of liquid paraffin (1ml/kg body weight, p.o) test group (group III – IV) were administered orally 50, 100 and 200 mg/kg body weight the plant compounds respectively compound aqueous suspension daily once a day. Group VI received Silmeryn, the known hepato protective compound (Sigma chemical company, USA), at a dose of 100mg/kg, p.o. along with CCI . Treatment duration was 14 days, dosage of CCI . was administered as 30% solution in liquid paraffin for every 72 hrs. Animals ware sacrificed 48 hrs after the last injection. Blood was collected, allowed to clot and serum was separated. Liver was dissected out used for biochemical studies.
Hepatoprotective activity method
In the pretreatments studies, animals were divided into six groups with 6 animals in each groups. Group I was served as normal control. Group II was CCI .control and group III, IV and were treated with plant extracts at three different doses 50, 100 and 200 mg/kg and group (VI) received an standard drug silymerin at a dose of 50ml/kg body weight, respectively.
Biochemical assay
Aspar tate amino transferase (AST), Alanine amino transferase (ALT) were determined. Liver tissue were homogenized on ice in 0.15M KCI. The homogenates were centrifuged at 300 rpm for 15 min at 4°C and the supernatants were taken for lipid per oxidation assay.
Results and Discussion
The present study report the effect of Cassia fistula fruits extracts on CCI .induced hepatic damage in albino rats. Three different doses of the extract was tried on CCI .inducded rats to observe AST, ALP and AIT parameter, from the result, it appears that maximum activity of dose III was quit comparable to the silymerin standard drug, which shows 24.64±1.28 of the total serum.
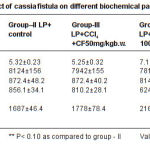 |
Table 2: Protective effect of cassia fistula on different biochemical parameters in the liver of rats Click here to View table |
Histo pathological findings as shown in figure (1) revealed the reduction of inflammation in normal central vein and hepato cytes to the normal Roy et al., (2006) have repor ted that decoction of fruit of Cassia fistula to cure jaundice within three days. The results present in table 1 and 2 revealed the decrease in ser um transaminase which may be due to stabilization of plasma membrane and hepato protection caused by plant extracts. In the present study it was noticed that 200mg/kg body weight dose of Cassia fistula gave total lipid value similar to the normal rat. The result are quite similar to the nor mal rat The results are quite similar as reported earlier by pradeep et al., (2005) who have noticed the recovery of hepatocytes damage caused by CCI . treatment after the Cassia fistula stage from the results of table (2), it appeared that protein was found to be significantly less in group II and III as compared to the control group I regarding extracts. Therefore the present finding suggests a triglycerides in liver tissue, it was increased in CCI .treated rats. Which got decreased to the normal level. Results as 200mg dosage were found to be quite effective which was similar to the silymerin. (P<0.05) hepatoprotective effect of Cassia fistula ethanolic extract against CCI .hepatocyte damage caused to the liver cells.
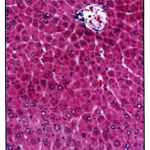 |
Figure 1: Shows normal hepatocytes and control vein after treatment with 200mg kg wt. (H.E. × 450) Click here to View figure |
References
- Wolf , P.L., Biochemical diagnosis of liver diseases. Indian J Clin Biochem, 14: 58-59 (1999).
- Anonymous., The wealth of India, vol – III, publication and information Directorate (CSIR), New Delhi, 337-343 (1992).
- Kirtikar, R.and Basu B.A., Indian medicinal plants, Vol – II, 2nd Edi. Periodical Exports book agency (1991).
- Yadava N. and verma V, A new biological active flavone glycoside from the seeds of Cassia fistula (Linn.) J.Asian Nat Brod Res.2003: 57-61(s).(2003).
- Roy, Chanchal Kamath jagadish, V and Asad Mohammad, Hepatoprotective activity of Psidium guava linn leaf extract. Indian J Exp Bio, 44: 305:311 (2006).
- Pradeep , Victor Rajmohan, C.Gobi Anand K.and Karthikeyan, S., Effect of pretreatment of Cassia fistula Linn. Leaf extract against subcute CCI .induced hepatotoxicity in rats. Indian J exp. S Biol, 43: 526-530 (2005).







