Manuscript accepted on :September 03, 2009
Published online on: 16-11-2015
Plagiarism Check: Yes
Pramilla Sah* and Vanita Kachhawaha
Department of Chemistry, J.N.V. University, Jodhpur - 342 005 India.
Abstract
The Mannich bases 2–phenyl–3–(1’H–morpholino/piperidino/ diphenylamino–methyl–2’–alkyl benzimidazolo–6/6,8–disubstituted quinazolin–4(3H)–ones were synthesized by treating 2–phenyl–2’–methyl/ethyl benzimidazolo–6/6,8–disubstituted quinazolin–4(3H)–ones with formaldehyde and appropriate secondary amines. The synthesized compounds were then evaluated for their insecticidal activity against Tribolium confusum, the host being jawar seeds. The survival percentage of the beetle reduced significantly and the development period of the insect was also prolonged between 10–14 days on an average by using 1.0, 2.5 and 5.0 ml/100 gm of the host seed by these Mannich bases.
Keywords
Mannich bases; Tribolium confusum; benzimidazolyl quinazolinones
Download this article as:| Copy the following to cite this article: Sah P, Kachhawaha V. Synthesis and Pharmacological Study of Some Mannich Bases of Benzimidazolyl Quinazolinones Against Tribolium Confusum. Biomed Pharmacol J 2009;2(2) |
| Copy the following to cite this URL: Sah P, Kachhawaha V. Synthesis and Pharmacological Study of Some Mannich Bases of Benzimidazolyl Quinazolinones Against Tribolium Confusum. Biomed Pharmacol J 2009;2(2).Available from: http://biomedpharmajournal.org/?p=742 |
Introduction
Quinazoline nucleus is endowed with various pharmaceutical applications e.g. used in the treatment of leprosy and mental disorders, as anticonvulsants, analgesic and antimicrobial agents.1–5 Methaquolone. Pyrazosin and Quinethazone are drugs that possess antihypertensive, antidiuretic and anticoagulant activities6. A number of Mannich bases have also been associated with a broad spectrum of biological activities i.e. antimicrobial, anti–inflammatory, antifilarial, antifungal etc.7–10 Recent reports have shown substituted benzimidazoles to be effective in the treatment of cancer, HIV–I (in–vitro) infections, as antihelmintic and therapeutic agents.8–15
Insects cover more than ¾ of the entire world fauna. It is roughly estimated that the animal loss due to the various pests affecting our crops is about 1500 crores per year, consequently their effect as carrier of disease is in no way less than their attack on crops. Tribolium confusum is considered as pest of gram flour, milled cereal products and stored commodities. For an efficient control the life cycle, host complex, mode of feeding and breeding play an important role.16–18
Large number of organic insecticides are available like DDT, BHC etc. The present study hence includes the synthesis and insecticidal activity of some Mannich bases incorporated with the two bioactive nuclei against the jawar seeds infected with this pest.
Experimental
Melting points were taken in open capillaries in an electrical ‘Neolab’ apparatus and are uncorrected. IR spectra were recorded on Schimadzu 8101 A spectrophotometer. 1HPMR in DMSO and CDCl3 on a Brucker DPX 300 MHz spectrophotometer. Mass was recorded on a JEOL SX 102/DA–6000 mass spectrometer.
Synthesis of monobromo and dibromo anthranilic acids
There were prepared by known procedure.19
Synthesis of N–Benzoyl–4–substituted–4/6–disubstituted anthranilic acids
The method of Dash et. al.20 and Reddy et. al.21 was followed.
2–phenyl–6/6,8–disubstituted benzoxazinones
0.015 mol of N–benzoyl–4–substituted/4,6–disubstituted anthranilic acids were refluxed for 30 min. in presence of acetic anhydride (10 ml). The solid mass which separated on cooling was recrystallized by repeated washing with petroleum ether (60–80°).
3a R1 = R2 = H, Yield 75%, M.P. °C 120°
3b R1 = Br, R2 = H, Yield 75%, M.P. °C 150°
3c R1 = Br, R2 = Br, Yield 75%, M.P. °C 160°
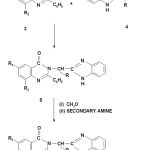
2–Amino methyl/ethyl benzimidazoles (4)
These were synthesized by the process of Cescon and Day.22
2–phenyl–(2’–methyl/ethyl benzimidazolo)–6/6,8–disubstituted quinazolin–
4(3H)–ones–(5a–5f)
Equimolar ratio of 2–aminomethyl benzimidazole (4a) and 2–phenyl benzoxazinone (3a) were refluxed in pyridine (10 ml) for 6 hrs. The solution was cooled, poured into ice water and neutralized with con. HCl (11.5 N). The solid which separated out was filtered, dried and recrystallized with ethanol. Similarly the other derivatives (5b–5f) were synthesized. Their physical and analytical data are given in Table I.
2–phenyl–3–(1’H–morpholino/piperidino/diphenylamino–methyl–2’–alkyl
benzimidazolo)–6/6,8–dibromo substituted quinazolin–4 (3H)–ones (6a–6l)
1 ml of 37% formaldehyde and 0.01% mole of morpholine was added to 0.01 mole of 5a in 5 ml ethanol with constant stirring. A turbid solution so obtained soon became clear on warming on a water bath for 2 minutes. It was left overnight at room temperature. The Mannich base so obtained was recrystallised from chloroform petroleum ether (60–80%) (1:1) ratio. Other Mannich bases were prepared similarly. The physical and analytical data is given in Table 2.
Table 1 : Physical And Analytical Data Of 2–Phenyl–(2’–Methyl/Ethyl Benzimidazolo)–6/6,8–Disubstituted Quinazolin–4(3h)–Ones.
| Compd. | Yield (%) | M.P. ° | Molecular Formula | Analysis Found (Calcd.) % | ||
| C | H | N | ||||
| 5a | 65 | 90 | C22H16N4O (352) | 74.77
(74.99) |
4.52
(4.54) |
15.88
(15.90) |
| 5b | 60 | 100 | C23H18N4O (366) | 75.42
(75.40) |
5.10
(4.91) |
15.40
(15.31) |
| 5c | 60 | 100 | C22H15N4OBr (431) | 61.35
(61.25) |
3.47
(3.48) |
13.05
(12.99) |
| 5d | 55 | 110 | C23H17N4OBr (445) | 62.05
(62.02) |
3.78
(3.82) |
12.54
(12.58) |
| 5e | 65 | 120 | C22H14N4OBr2 (510) | 51.78
(51.76) |
2.76
(2.74) |
10.79
(10.98) |
| 5f | 65 | 130 | C23H16N4OBr2 (524) | 52.75
(52.67) |
3.04
(3.05) |
10.71
(10.68) |
Table 2 : Characteristic Data Of 2–Phenyl–3–(1’–H–Morpholino/Piperidino/Diphenylamino–Methyl–2’–Alkyl Benzimidazolo)–6/6,8–Disubstituted Quinazolin–4(3h)–Ones
| Compd. | R | R1 | R2 | R3 | M.P.° | Molecular Formula | Analysis of Nitrogen Found
(Calcd.) % |
| 6a | H | H | H | Morpholino | 110 | C27H25N5O2 (451) | 15.56
(15.52) |
| 6b | H | H | H | Piperidino | 110 | C28H27N5O (449) | 15.61
(15.59) |
| 6c | H | H | H | Diphenylamino | 120 | C35H27N5O (533) | 13.25
(13.13) |
| 6d | CH3 | H | H | Morpholino | 115 | C28H27N5O2 (465) | 15.08
(15.05) |
| 6e | CH3 | H | H | Piperidino | 120 | C29H29N5O (463) | 15.21
(15.11) |
| 6f | CH3 | H | H | Diphenylamino | 125 | C36H29N5O (547) | 12.78
(12.79) |
| 6g | H | Br | H | Piperidino | 180 | C28H26N5OBr (558) | 12.57
(12.54) |
| 6h | CH3 | Br | H | Diphenylamino | 130 | C36H28N5OBr (626) | 11.27
(11.18) |
| 6i | H | Br | Br | Morpholino | 130 | C27H23N5O2Br2 (609) | 11.51
(11.49) |
| 6j | H | Br | Br | Piperidino | 190 | C28H25N5OBr2 (607) | 11.47
(11.53) |
| 6k | CH3 | Br | Br | Diphenylamino | 140 | C36H27N5OBr2 (705) | 10.13
(9.92) |
| 6l | CH3 | Br | Br | Morpholino | 135 | C28H25N5O2Br2 (623) | 11.32
(11.23) |
Yield between 45 – 65%
Table 3 : Spectral Data Of The Newly Synthesized Compounds
Stretching), 3300 (–NH)5g 1665 (C=O), 1620 (C=N), 1327 (C–N), 2890, 3035 (–CH, Stretching),3310 (–NH), 740 (C–Br)6a 1665 (C=O), 1620 (C=N), 1318 (C–N), 2895, 3030 (–CH, Stretching)6e 1670 (C=O), 1625 (C=N), 1315 (C–N), 2885, 3030 (–CH, Stretching)6k 1660 (C=O), 1620 (C=N), 1320 (C–N), 2890, 3035 (–CH, Stretching), 750 (C–Br)1HPMR(DMSO + CDCl3, d ppm) 5a : 4.34 (2H, s, N–CH2–C), 6.87 (1H, s, NH), 7.52 – 8.11 (13H, m, ArH)6a : 4.32 (2H, s, N–CH2–C), 4.75 (2H, s, N–CH2–N), 2.91 (4H, t, CH2–N–CH2), 3.33 (4H, t, CH2–O–CH2), 7.56 – 8.01 (13H, m, ArH)6b : 4.35 (2H, s, N–CH2–C), 4.70 (2H, s, N–CH2–N), 2.75 (4H, t, CH2–N–CH2), 1.70 (6H, s, CH2– CH2–CH2), 7.48 – 7.96 (13H, m, ArH)6c : 4.35 (2H, s, N–CH2–C), 4.82 (2H, s, N–CH2–N), 7.38 – 8.25 (23H, m, ArH)Mass Spectra 6c MS+ 533, m/z, 351, 235, 221 (100%), 193, 182, 180, 130, 118, 116, 103
Evaluation of insecticidal activity
Tribolium confusum was reared on whole wheat flour supplemented with brewer’s yeast at 30 ± 2 °C and 70 ± 5% relative humidity in the laboratory by following the known procedure.23The insecticidal activity against Tribolium confusum was evaluated in terms of percentage adult mortality and percentage adult emergence on the host jawar grains.
Adult Mortality
To study the effect of the different synthesized Mannich bases on the adult mortality of Tribolium confusum, ten newly emerged adults were introduced in the culture tubes containing ten grams of chemically treated jawar grains. The stock solution was made by mixing 5 gms of the synthesized compound with 95 ml of benzene. This solution was applied on jawar grains at three different doses (levels) i.e. 1.0, 2.5 and 5.0 ml/100 gms of jawar grains respectively. It was kept for sometime till a homogeneous mass was formed. Culture tubes containing the adults (ten in number) of Tribolium confusum were covered with a muslin cloth and then tied with a rubber band. The tubes were placed in an incubator at 28 ± 2°C and 75 ± 5% R.H. The mortality percentage of adults in their number was observed after ten days.Each experiment was performed in triplicate. The percentage of adult mortality at the three concentration levels are given in Table 4.
 |
Scheme 4 : Adult Mortality Of Tribolium Confusum On Jawar Seeds Treated With Mannich Bases Of Benzimidazolyl Quinazolinones.
|
Adult Emergence
Twenty newly hatched larvae were collected from the stock culture of Tribolium confusum maintained in wheat flour were carefully transferred to culture tubes containing jawar grains treated with different Mannich bases to study the development period. The required amount of the compound was mixed with jawar grains at concentration levels of 1.0, 2.5 and 5 ml/100 gm, respectively. The culture tubes were covered with a muslin cloth and tied with a rubber band. The tubes were placed in an incubator at 28 ± 2°C and 75 ± 5% R.H. The observations were made on period required for adult emergence till the emergence of last adult from 5th to 14th day and the emerged beetles were removed to prevent further breeding. Each experiment was performed in triplicate, and the percentage of adult emergence has been given in Table 5.
Statistical Analysis
Analysis of variance (ANOVA) was also performed to see the significance of treatment given. [4A, 5A]
Insecticidal Activity
The percentage of adult mortality (Table IV) at 1 ml/100 gm seeds in seeds treated with 2–phenyl–3–(1’H–morpholinomethyl–2’–methyl benzimidazolo)–6,8 dibromo quinazolin–4(3H)–one was maximum in 6i (26.7%) while three derivatives 6a, 6c, 6g showed the minimum value of 6.67%.At the concentration of 2.5 ml/100 gm the adult mortality percentage increased upto 33.33% in case of 6d, 6e, 6k and 6i in the seeds treated with the compounds as compared to the control (0%).With the further increase in the concentration @ 5 ml/100 gm the percentage of adult mortality was much higher i.e. 60% (6l).The highest mortality percentage was observed when morpholine was one of the substituent in the synthesis of the Mannich bases. Furthermore presence of a bromo group also effectively increased the mortality rate of the insect at all the concentrations.It is also apparent that the percentage of mortality showed a random increase with an increase in the concentration, while ANOVA data show CD at 5% to be 5.96.
Adult Emergence
The adult emergence of Tribolium confusum on jawar seeds treated with Mannich bases (Table V) in percentage @ 1 ml/100 gm was 10% (6i, 6j, 6k and 6l) and ranged upto 33.3% for 2–phenyl–3–(1’H–diphenylamino– methyl–2’–ethyl benzimidazolo)–6–bromo–quinazolin–4(3H) –one (6h).At a concentration of 2.5 ml/100 gm the adult emergence percentage increased and ranged between 13.33% (6i and 6k) to 46.67% in case of 2–phenyl–3–(1’H–piperidino methyl–2’–ethyl benzimidazolo)–quinazolin–4 (3H)–one (6e).With further increase in concentration at the rate of 5 ml/100 gm, the highest adult emergence percentage was recorded as 53.33% (6e) and lowest was 16.67% (6i).All the treatments caused lower adult emergence in comparison to the control (90%) with all the three doses. The ANOVA analysis showed the CD at 5% to be 5.11. Therefore the test chemicals greatly effected the life cycle of T. confusum.Various methods have been published to minimize the problem.24–25Some synthetic compounds like the benzimidazole derivatives have been found to be effective against Tribolium confusum infesting house hold grain sorghum.26 The above data further highlight the importance of heterocyclic compounds and hence these Mannich bases could be useful as possible insecticidal agents.
Acknowledgement
The authors are thankful to the Director SAIF, CDRI, Lucknow for the spectral analysis, the Head Department of Zoology, J.N.V. University, Jodhpur for the biological screening of the compound and the Statistical Division of Central Arid Zone Research Institute, Jodhpur.
Table 4 : Adult Mortality Of Tribolium Confusum On Jawar Seeds Treated With Mannich Bases Of Benzimidazolyl Quinazolinones.
| % Adult Mortality | ||||||||||||
| Compd. | 1.0 ml/100g | 2.5 ml/100g | 5.0 ml/100g | |||||||||
| R1 | R2 | R3 | Mean | R1 | R2 | R3 | Mean | R1 | R2 | R3 | Mean | |
| 6a | 10 | 10 | 0 | 6.67 | 10 | 10 | 20 | 13.33 | 20 | 20 | 10 | 16.67 |
| 6b | 10 | 10 | 10 | 10 | 10 | 20 | 20 | 15.67 | 30 | 30 | 40 | 33.33 |
| 6c | 10 | 0 | 10 | 6.67 | 10 | 20 | 30 | 20 | 30 | 40 | 50 | 40 |
| 6d | 20 | 20 | 10 | 16.7 | 30 | 40 | 30 | 33.33 | 50 | 40 | 40 | 43.33 |
| 6e | 20 | 20 | 20 | 20 | 30 | 30 | 40 | 33.33 | 50 | 50 | 50 | 50 |
| 6f | 20 | 10 | 20 | 16.7 | 30 | 20 | 20 | 23.33 | 40 | 40 | 50 | 43.33 |
| 6g | 10 | 10 | 0 | 6.67 | 20 | 20 | 20 | 20 | 30 | 40 | 40 | 36.67 |
| 6h | 10 | 10 | 20 | 13.3 | 20 | 20 | 20 | 20 | 30 | 30 | 40 | 33.33 |
| 6i | 20 | 30 | 30 | 26.7 | 30 | 30 | 30 | 30 | 40 | 50 | 50 | 46.67 |
| 6j | 20 | 20 | 20 | 20 | 30 | 30 | 30 | 30 | 50 | 50 | 60 | 53.33 |
| 6k | 10 | 10 | 20 | 13.3 | 30 | 30 | 40 | 33.33 | 50 | 50 | 50 | 50 |
| 6l | 20 | 10 | 10 | 13.3 | 40 | 30 | 30 | 33.33 | 60 | 60 | 60 | 60 |
| CONTROL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
4A : Anova : Two–Factor with Replication
6a
| Summary | 1.0 ml/100 g | 2.5 ml/100 g | 5.0 ml/100 g | Total |
| Count | 3 | 3 | 3 | 9 |
| Sum | 20 | 40 | 50 | 110 |
| Average | 6.67 | 13.33 | 16. 67 | 12.22 |
| Variance | 33.33 | 33.33 | 33.33 | 44.44 |
6b
| Summary | 1.0 ml/100 g | 2.5 ml/100 g | 5.0 ml/100 g | Total |
| Count | 3 | 3 | 3 | 9 |
| Sum | 39 | 50 | 100 | 180 |
| Average | 10 | 16.67 | 33.33 | 20 |
| Variance | 0 | 33.33 | 33.33 | 125 |
6c
| Summary | 1.0 ml/100 g | 2.5 ml/100 g | 5.0 ml/100 g | Total |
| Count | 3 | 3 | 3 | 9 |
| Sum | 20 | 60 | 120 | 200 |
| Average | 6.67 | 20 | 40 | 22.22 |
| Variance | 33.33 | 100 | 100 | 269.44 |
6d
| Summary | 1.0 ml/100 g | 2.5 ml/100 g | 5.0 ml/100 g | Total |
| Count | 3 | 3 | 3 | 9 |
| Sum | 50 | 100 | 130 | 280 |
| Average | 16.67 | 33.331 | 43.33 | 31.11 |
| Variance | 33.33 | 33.33 | 33.33 | 161.11 |
6e
| Summary | 1.0 ml/100 g | 2.5 ml/100 g | 5.0 ml/100 g | Total |
| Count | 3 | 3 | 3 | 9 |
| Sum | 60 | 100 | 150 | 310 |
| Average | 20 | 33.33 | 50 | 34.44 |
| Variance | 0 | 33.33 | 0 | 177.78 |
6f
| Summary | 1.0 ml/100 g | 2.5 ml/100 g | 5.0 ml/100 g | Total |
| Count | 3 | 3 | 3 | 9 |
| Sum | 50 | 70 | 130 | 250 |
| Average | 16.67 | 23.33 | 43.33 | 27.78 |
| Variance | 33.33 | 33.33 | 33.33 | 169.44 |
6g
| Summary | 1.0 ml/100 g | 2.5 ml/100 g | 5.0 ml/100 g | Total |
| Count | 3 | 3 | 3 | 9 |
| Sum | 20 | 60 | 110 | 190 |
| Average | 6.67 | 20.00 | 36.67 | 21.11 |
| Variance | 33.33 | 0.00 | 33.33 | 186.11 |
6h
| Summary | 1.0 ml/100 g | 2.5 ml/100 g | 5.0 ml/100 g | Total |
| Count | 3 | 3 | 3 | 9 |
| Sum | 40 | 60 | 100 | 200 |
| Average | 13.33 | 20.00 | 33.33 | 22.22 |
| Variance | 33.33 | 0.00 | 33.33 | 94.44 |
6i
| Summary | 1.0 ml/100 g | 2.5 ml/100 g | 5.0 ml/100 g | Total |
| Count | 3 | 3 | 3 | 9 |
| Sum | 80 | 90 | 140 | 310 |
| Average | 26.67 | 30.00 | 46.67 | 34.44 |
| Variance | 33.33 | 0.00 | 33.33 | 102.78 |
6j
| Summary | 1.0 ml/100 g | 2.5 ml/100 g | 5.0 ml/100 g | Total |
| Count | 3 | 3 | 3 | 9 |
| Sum | 60 | 90 | 160 | 310 |
| Average | 20 | 30 | 53.33 | 34.44 |
| Variance | 0 | 0 | 33.33 | 227.78 |
6k
| Summary | 1.0 ml/100 g | 2.5 ml/100 g | 5.0 ml/100 g | Total |
| Count | 3 | 3 | 3 | 9 |
| Sum | 40 | 100 | 150 | 290 |
| Average | 13.33 | 33.33 | 50.00 | 32.22 |
| Variance | 33.33 | 33.33 | 0.00 | 269.44 |
6l
| Summary | 1.0 ml/100 g | 2.5 ml/100 g | 5.0 ml/100 g | Total |
| Count | 3 | 3 | 3 | 9 |
| Sum | 40 | 100 | 180 | 320 |
| Average | 13.33 | 33.33 | 60.00 | 35.56 |
| Variance | 33.33 | 33.33 | 0.00 | 427.78 |
Total
| Summary | 1.0 ml/100 g | 2.5 ml/100 g | 5.0 ml/100 g |
| Count | 36 | 36 | 36 |
| Sum | 510 | 920 | 1520 |
| Average | 14.17 | 25.56 | 42.22 |
| Variance | 53.57 | 71.11 | 143.49 |
Anova
| Source of variation | df | MS | F |
| Chemical | 11 | 516.08 | 18.58 |
| Concentration | 2 | 7167.59 | 258.03 |
| Chemical* Conc. | 22 | 77.69 | 2.80 |
| Error | 72 | 27.78 | |
| Total | 107 |
SEM = 3.04
CD AT 5% = 5.96
Table 5 : Adult Emergence of Tribolium Confusum on Jawar Seeds Treated With Mannich Bases of Benzimidazolyl Quinazolinones.
| % Adult emergence | ||||||||||||
| Compound | 1.0 ml/100g | 2.5 ml/100g | 5.0 ml/100g | |||||||||
| R1 | R2 | R3 | Mean | R1 | R2 | R3 | Mean | R1 | R2 | R3 | Mean | |
| 6a | 10 | 10 | 20 | 13.33 | 20 | 20 | 10 | 16.67 | 30 | 30 | 20 | 26.67 |
| 6b | 10 | 20 | 20 | 16.7 | 30 | 30 | 20 | 26.67 | 40 | 40 | 50 | 43.33 |
| 6c | 20 | 20 | 30 | 23.3 | 30 | 30 | 30 | 30 | 40 | 50 | 50 | 46.67 |
| 6d | 30 | 20 | 30 | 26.7 | 40 | 40 | 50 | 43.33 | 50 | 50 | 50 | 50 |
| 6e | 30 | 30 | 30 | 30 | 50 | 50 | 40 | 46.67 | 50 | 50 | 60 | 53.33 |
| 6f | 30 | 30 | 20 | 26.7 | 40 | 40 | 40 | 40 | 50 | 50 | 50 | 60 |
| 6g | 20 | 20 | 20 | 20 | 30 | 30 | 30 | 30 | 40 | 40 | 40 | 40 |
| 6h | 30 | 30 | 40 | 33.3 | 40 | 30 | 40 | 36.67 | 50 | 40 | 50 | 46.67 |
| 6i | 10 | 10 | 10 | 10 | 10 | 10 | 20 | 13.33 | 20 | 20 | 10 | 16.67 |
| 6j | 10 | 10 | 10 | 10 | 20 | 20 | 20 | 20 | 30 | 30 | 30 | 30 |
| 6k | 10 | 10 | 10 | 10 | 10 | 10 | 20 | 13.33 | 20 | 20 | 30 | 23.33 |
| 6l | 10 | 10 | 10 | 10 | 20 | 20 | 10 | 16.67 | 30 | 20 | 30 | 26.67 |
| CONTROL | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 90 |
5A : Anova : Two–Factor with Replication
6a
| Summary | 1.0 ml/100 g | 2.5 ml/100 g | 5.0 ml/100 g | Total |
| Count | 3 | 3 | 3 | 9 |
| Sum | 40 | 50 | 80 | 170 |
| Average | 13.33 | 16.67 | 26. 67 | 18.89 |
| Variance | 33.33 | 33.33 | 33.33 | 61.11 |
6b
| Summary | 1.0 ml/100 g | 2.5 ml/100 g | 5.0 ml/100 g | Total |
| Count | 3 | 3 | 3 | 9 |
| Sum | 50 | 80 | 130 | 260 |
| Average | 16.67 | 26.67 | 43.33 | 28.89 |
| Variance | 33.33 | 33.33 | 33.33 | 161.11 |
6c
| Summary | 1.0 ml/100 g | 2.5 ml/100 g | 5.0 ml/100 g | Total |
| Count | 3 | 3 | 3 | 9 |
| Sum | 70 | 90 | 140 | 300 |
| Average | 23.33 | 30.00 | 46.67 | 33.33 |
| Variance | 33.33 | 0.00 | 33.33 | 125.00 |
6d
| Summary | 1.0 ml/100 g | 2.5 ml/100 g | 5.0 ml/100 g | Total |
| Count | 3 | 3 | 3 | 9 |
| Sum | 80 | 130 | 150 | 360 |
| Average | 26.67 | 43.33 | 50 | 40 |
| Variance | 33.33 | 33.33 | 0 | 125 |
6e
| Summary | 1.0 ml/100 g | 2.5 ml/100 g | 5.0 ml/100 g | Total |
| Count | 3 | 3 | 3 | 9 |
| Sum | 90 | 140 | 160 | 390 |
| Average | 30 | 46.67 | 53.33 | 43.33 |
| Variance | 0 | 33.33 | 33.33 | 125 |
6f
| Summary | 1.0 ml/100 g | 2.5 ml/100 g | 5.0 ml/100 g | Total |
| Count | 3 | 3 | 3 | 9 |
| Sum | 80 | 120 | 150 | 350 |
| Average | 26.67 | 40.00 | 50.00 | 38.89 |
| Variance | 33.33 | 0.00 | 0.00 | 111.11 |
6g
| Summary | 1.0 ml/100 g | 2.5 ml/100 g | 5.0 ml/100 g | Total |
| Count | 3 | 3 | 3 | 9 |
| Sum | 60 | 90 | 120 | 270 |
| Average | 20 | 30 | 40 | 30 |
| Variance | 0 | 0 | 0 | 75 |
6h
| Summary | 1.0 ml/100 g | 2.5 ml/100 g | 5.0 ml/100 g | Total |
| Count | 3 | 3 | 3 | 9 |
| Sum | 100 | 110 | 140 | 350 |
| Average | 33.33 | 36.67 | 46.67 | 38.89 |
| Variance | 33.33 | 33.33 | 33.33 | 61.11 |
6i
| Summary | 1.0 ml/100 g | 2.5 ml/100 g | 5.0 ml/100 g | Total |
| Count | 3 | 3 | 3 | 9 |
| Sum | 30 | 40 | 50 | 120 |
| Average | 10 | 13.33 | 16.67 | 13.33 |
| Variance | 0 | 33.33 | 33.33 | 25.00 |
6j
| Summary | 1.0 ml/100 g | 2.5 ml/100 g | 5.0 ml/100 g | Total |
| Count | 3 | 3 | 3 | 9 |
| Sum | 30 | 60 | 90 | 180 |
| Average | 10 | 20 | 30 | 20 |
| Variance | 0 | 0 | 0 | 75 |
6k
| Summary | 1.0 ml/100 g | 2.5 ml/100 g | 5.0 ml/100 g | Total |
| Count | 3 | 3 | 3 | 9 |
| Sum | 30 | 40 | 70 | 140 |
| Average | 10 | 13.33 | 23.33 | 15.56 |
| Variance | 0 | 33.33 | 33.33 | 52.78 |
6l
| Summary | 1.0 ml/100 g | 2.5 ml/100 g | 5.0 ml/100 g | Total |
| Count | 3 | 3 | 3 | 9 |
| Sum | 30 | 50 | 80 | 160 |
| Average | 10 | 16.67 | 26.67 | 17.78 |
| Variance | 0 | 33.33 | 33.33 | 69.44 |
Total
| Summary | 1.0 ml/100 g | 2.5 ml/100 g | 5.0 ml/100 g |
| Count | 36 | 36 | 36 |
| Sum | 690 | 1000 | 1360 |
| Average | 19.17 | 27.78 | 37.78 |
| Variance | 82.14 | 149.21 | 160.63 |
Anava
| Source of variation | df | MS | F |
| Chemical | 11 | 1039.31 | 51.02 |
| Concentration | 2 | 3123.15 | 153.32 |
| Chemical* Conc. | 22 | 37.29 | 1.83 |
| Error | 72 | 20.37 | |
| Total | 107 |
SEM = 2.61
CD AT 5% = 5.11
References
- El–Helpy, A.G., J. Pharm. Sci., 14, 193 (1994).
- Kanna, R., Saxena, A.K., Srivastava, V.K. and Shankar, K., Indian J. Chem., 29B, 1056 (1990).
- Kant, P. and Saxena, R.K., Indian J. Hetero. Chem., 12, 315 (2003).
- Alagarswamy, V., Muthu Kumar, V., Pavalaram, N., Vasanthanathan, P. and Revathi, P., Biol. Pharm. Bull., 26(4), 557 (2003).
- Sharma, B.P., Lakhan, R. and Singh, B., J. Indian Chem. Soc., 82, 651 (2005).
- Swift, J.G., Dickens, E.A. and Beacker, B.A., Arch. Int. Pharmacodyn., 128, 112 (1960).
- Varma, R.S., Shukla, A., Fatma, N. and Chatterjee, R.K., Indian J. Chem., 32B, 347 (1993).
- Ghantwal, S.R. and Samant, S.D., J. Indian Chem. Soc., 77, 100 (2000).
- Gaikwad, N.J. and Gautam, P., Indian J. Hetero. Chem., 12(2), 181 (2002).
- Satayanarayana, D., Prakash Reddy, R.K., Ramana, M.V., Subrahmanyam, E.V.S., Himaja, M. and Kalluraya, B., Indian J. Hetero. Chem., 10(1), 45 (2000).
- Walmaley, D.L., Drysdale, M.J., Northfield, C.J. and Fromont, C., PCT Int. Appl. WO 134, 318 (2006) Chem. Abs. 146 : 81861q.
- Rida, S.M., Cet Hawash, S.A.M., Fahmy, H.T.Y., Hazza, A.A. and El–Meligy, M.M.M., Archives Pharmacol. Research, 29(10), 826 (2006).
- Mavrova, A.Ts., Anichina, K.K., Vuchev, D.I., Tsenov, J.A., Denkova, P.S., Kondeva, M.S. and Micheva, M.K., Eur. J. Med. Chem., 41(12), 1412 (2006).
- Reuton, P. Maddaford, S., Rakhit, S. and Andrews, J., PCT. Int. Appl. WO 17, 764 (2007) Chem. Abs., 146 : 229352x.
- Parmar, S. and Sah, P., Indian J. Hetero. Chem., 16, 367 (2007).
- Krishnamurti, B. Mysore Agric, J. 22, 40–45 (1943).
- Prakash, A., Pasalu, I.C. and Rao, J., Trop. Stored Prod. Inf., 47, 15 (1984).
- Sokoloff, A., The Biology of Tribolium, Oxford Univ. Press, London, 1, 300 (1972).
- Wheeler, A.S. and Oats, W.M., J. Am. Chem. Soc., 32, 770 (1910).
- Dash, B., Dova, E.K. and Panda, C.S., J. Indian Chem. Soc., LV11, 835 (1980).
- Reddy, G.S. and Reddy, K.K., Indian J. Chem., 16B, 1109 (1978).
- Cescon, L.A. and Day, A.R., J. Org. Chem., 27, 581 (1962).
- Uehara, M., Watanabe, M., Kimura, M., Morimoto, M. and Yoshida, M., Eur. Pat. Appl., 97, 932 (2001).
- Anonymous, Annual Tech. Report Central Rice Research Institute, Cuttack (India), 167–169 (1969).







