Manuscript accepted on :August 26, 2009
Published online on: 19-11-2015
Plagiarism Check: Yes
Sudhaldev Mohapatra* and C. B. Jha
Department of Rasa Shastra, Faculty of Ayurveda, Institute of Medical Science, Banaras Hindu University, Varanasi - 221 005 India. Corresponding Author E-mail:ras_dev@rediffmail.com
Abstract
Swarna Makshika [SM] an Ayurvedic raw material is a mineral that contains Cu, Fe & S as main ingredient. It is processed by following Ayurvedic ancient methodology and bhasma is obtained after confirming Ayurvedic quality control parameters like nischandratva, varitara, rekhapurna, niswadu, amlaparikshya etc. Then metallographic features of raw SM and Bhasma of SM with intermediary process are analyzed to know about the physicochemical nature. The raw SM shows the crystallinity, conchoidal structure and larger size of the grain, where as in bhasma crystalinity was not found and grain size reduced significantly. Also raw material shows similar pattern of darkness and brightness in the metallographic microscope where as in bhasma many different color and patterns are observed that may be due to presence of different compounds formed due to process effect. The observations are documented for its validation following same pharmaceutical procedure. The data generated could be the fingerprint for its standardization.
Keywords
Ayurvedic bhasmas; Swarna Makshika
Download this article as:| Copy the following to cite this article: Mohapatra S, Jha C. B. Metallographic Study of Ayurvedic Bhasmas- An Analytical Study of Swarna Makshika (Chalcopyrite). Biomed Pharmacol J 2009;2(2) |
| Copy the following to cite this URL: Mohapatra S, Jha C. B. Metallographic Study of Ayurvedic Bhasmas- An Analytical Study of Swarna Makshika (Chalcopyrite). Biomed Pharmacol J 2009;2(2). Available from: http://biomedpharmajournal.org/?p=942 |
Introduction
SM bhasma is used for treatment of anemia, anxiety, weakness, and cardiac problems etc. and also as a rejuvenator¹. In today’s era commercialization of Ayurvedic medicines hindered its quality and give birth of many questions regarding its safety and efficacy. Hence Standardization and Validation of bhasmas are the demand of time for its safe use in therapeutics which needs extensive analysis in different modern tools and techniques. With this view metallographic study is planned. Metallographic study reveals the structural characteristics of a metal/mineral or the phase orientations of an alloy in relation to its physicochemical properties.
Swarna makshika is processed (shodhana & marana) with lemon juices & shuddha gandhaka (sulphur) at about 900°c temp. till the features of bhasma like nischandratva, varitara, niswadu, amlaparikshya etc. are observed. Metallographic study of Swarna makshika discloses surface reaction process, to find out the mechanism of bhasma preparation, nature of compounds by observing the etching characteristics of the bhasma.
Swarna Makshika processing²
Raw SM is procured from Ayurvedic pharmacy of Banaras Hindu University and made into powder form. A clean iron kdhai (pan) was taken and heated on the charcoal furnace, then SM powder was poured and subjected to intense heat with frequent addition of lemon juice till the sulphur fume stops and red color appeared. Then equal amount shoddhita SM and shuddha gandhaka were triturated with lemon juice, small pellets of uniform size and thickness were prepared, dried in sun light. Dried pellets were kept inside the sarava samputa (one earthen casserole is inverted with another earthen casserole); joint was sealed by rag & mud (kapadmiti) for seven times and dried. Properly sealed and dried samputa was subjected to puta system of heating with four kg cow dung cake. The process was repeated taking equal amount of shuddha gandhaka in first firing and half the amount of shuddha gandhaka in succeeding firings, in comparison to shuddha SM and the desired quality of bhasma was achieved in 09 firings.
The bhasma obtained from the above process was taken for Metallographic study.
Metallographic Study³
Materials and Method
Materials
Samples
Raw Swarna makshika (1-2 gm)
Shoddhita Swarna makshika (1-2 gm)
Swarna makshika bhasma (1-2 gm)
Chemicals
Geosyn Cold Mounting (GCM) compound & liquid
Grease & Aluminum powder
Nitric acid & Ferric chloride
Apparatus required
Mounting socket, Plane glass plate, Glass rod, Spatula, Petridish, Cotton, Applicator stick
Specially designed Grinding machine with Emery belt, Polishing machine, Metallurgical Electronic Microscope with attached Computer
Method
Preparation of Metallographic Specimen
Mounting
1 g of GCM compound and 500mg of sample to be analyzed were taken in a petridish, mixed uniformly by a clean glass rod. Clean and dry copper mounting socket smeared with grease in it’s internal surface was placed on a clean glass plate having greased surface. The mixture of the sample and GCM compound was poured into the socket then sufficient amount of GCM liquid was poured over the mixture. It was left as such for 2-3 min. When all the material inside the socket became solid it was removed, cleaned with soap & water then subjected for grinding.
Grinding
Grinding of the sample is done to cut a number of particles through the center exposing the internal structure of the particles and also to make it scratch free for metallographic examination.
Rough grinding
At first the surface of the sample was made plain and smooth with the help of a specially designed motor driven emery belt. During the grinding, water was added frequently to facilitate to lower the temperature of the grinding surface, which may alter the nature of the grain of the sample. Grinding was done till the flat appearance of the sample. Then the sample was washed thoroughly with soap and water.
Intermediate grinding
Graded emery papers of high quality, with respect to size and uniformity of the emery particles were used and each time paper was changed for each sample. First grade No. 1 grinding paper was used. The emery paper was kept on a clean and hard surface such as sheet of glass. Under normal pressure the specimen was gently drawn back and forth across the entire length of the paper, while being ground, the mounted sample was hold in such a way that the new finer scratches should remain approximately at the right angles to the older scratches produced by the rough grinding. It was completed when the coarse scratches are replaced by the finer scratches.
Fine grinding
The same procedure was adopted as previous with emery papers having increasing fineness. Here grade No. 000 papers were used. After the removal of the coarse scratches sample was subjected to polishing.
Polishing
Polishing of a metallographic specimen is done to obtain a scratch free surface by removing fine scratches introduced during the grinding operation.
Preliminary Polishing
The polishing operations are performed on polishing laps made up of bronze discs 8 to 10” in diameter, covered with an appropriate grade of polishing cotton cloth. The laps are motor driven and rotated in a horizontal plane. The disc is rotated at about 400 to 500 rpm. Sample is held firmly against the rotatory lap and moved back and forth.
Final polishing
In this process same operations are repeated with the use of more smooth velvet cloth
The sample is held with moderate pressure against the moving lap and moved in a counter direction to the direction of the rotation of the wheel. After this the sample is washed in running water, swabbed with wet cloth to remove the polishing materials, then rinsed smoothly by methyl alcohol wet swab then dried in warm air.
Etching
Etching is a process in which the required chemical reagent i.e. etchant is applied to the polished metallographic surface, to open the details of the metallographic structure. Different constituents in the mounted sample and different orientations of the grains are reacting differently with the etchant substances hence using different etchant different metallographic structures are revealed.
Pre etching treatment:
Before etching the mounted surface was cleaned with soap and running water care fully, made free from tarnish, like oily and greasy substances used during mounting procedure, to ensure the uniformity. Then swabbed with cotton and dried.
Etching Reagent
Particular etchants are used for particular metals, which exposes proper metallographic structural approach to the surface. For Iron 2% Nital and for Copper FeCl2 was used as etching reagent.
Concentration of Etchants
Concentration of the etchant is the most important factor for proper observation of grains. Low concentration of etchant is used to study the metallographic structures. During etching, due to the difference in the rate of dissolution of different crystallographic planes, well defined facets are developed which gives rise to a structure known as ‘oriented grain luster’ in which some of the grain sections appear bright and lustrous, where as others appear dark and dull.
Etching Time
It depends on the, particular metal, reagent and the magnification under which the sample to be observed. If etching is done for a longer period or less than the required time, then it is over etched or under etched. By this the metallographic structure is not properly observed. Over etching shows the deep pits, facets and coarse grain boundaries, where as under etching shows the faint structure and grain boundaries.
Process of Etching
Etching was done with the help of cotton soaked in etching solution that attacks the surface at a rate, which varies with the crystalline orientation of the surface. A clump of cotton was wrapped over an applicator stick and made thoroughly saturated with the required etching reagent. The specimen surface was vigorously swabbed by the above applicator for the appropriate time.
Microscopic Study of the Prepared Sample
The polished and etched sample was mounted on the glass slide with the help of plasticine. Then it was placed under a spring loaded specimen leveling device and pressed to be fixed and leveled on the glass slide. Microscope was focused on the surface of the sample at first observed under low power (10X) and then under high magnification (100 X, 200 X, 400 X). The whole cross section was observed minutely by moving the slide in X and Y direction. Then the image was transferred to the attached computer and the photographs were taken. The sample was first examined in a polished condition and then with its etching condition.
Metallographic figures showing Structural changes in Raw SM, Intermediary process and SM Bhasma:
Observations and Results
Figure1 shows Conchoidal structures of the grains of sulphur compounds of iron and copper when examined under the microscope in high magnification. The size of the grain was considerably larger.
 |
Figure 1: Microphotograph Showing Conchoidal Structure Of Raw Swarna Makshika (400x).
|
In Fig. 2 Breaking of the Conchoidal structure is observed in case of Shoddhita SM under high magnifications dark color of the grains of more sulphide as well as bright color of some oxide compounds of iron and copper were observed.
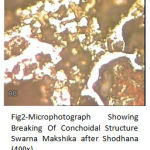 |
Figure 2: Microphotograph Showing Breaking Of Conchoidal Structure Swarna Makshika after Shodhana (400x).
|
From Fig. 3 to Fig. 5 shows the gradual formation of oxide and sulphide compounds of Iron and Copper, inter diffusion of surface compound towards the core, conversion of free metal to the compounds, and reduction in grain size in succeeding putas.
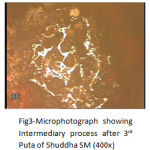 |
Figure 3: Microphotograph showing Intermediary process after 3rd Puta of Shuddha SM (400x).
|
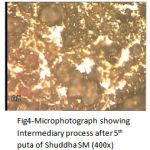 |
Figure 4: Microphotograph showing Intermediary process after 5th puta of Shuddha SM (400x).
|
 |
Figure 5 Microphotograph showing Intermediary process after 7th puta of Shuddha SM (400x).
|
The grain size of the Bhasma, in Fig. 6 was found significantly reduced, the orientations of the grains were also changed and the grains of more bright oxide compounds were found with some dark sulphide compounds
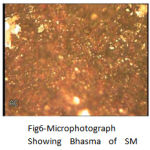 |
Figure 6: Microphotograph Showing Bhasma of SM.
|
When SM Bhasma is etched with Nital, more brightness in the sample (Fig. 7) is predominately observed darkness is observed when etched with ferric chloride (Fig. 8).
 |
Figure 7: Microphotograph Showing SM Bhasma etched with Nital.
|
 |
Figure 8: Microphotograph Showing SM Bhasma etched with Ferric chloride (400X).
|
Discussion
The chemical process of preparation of bhasma is the heterogeneous reaction occurring in two phases’ viz. solid – liquid phase and liquid – gas phase and the chemical reaction occurring during the bhasma preparation is controlled by the interfacial reaction between solid-liquid and liquid–gas [4].
After the unset of the oxidation and reduction process the bhasma particle on the surface is covered with the organometalic compounds i.e. various oxide and sulphide compounds of the metal present. Further progress of reaction depends on the environment provided i.e. particular temperature and pressure {quantum of heat, duration of heating, and svangasita (self cooling) period}[5].
When the bhasma is again subjected to trituration for next puta then there is uniform intermixing of surface and core compound occurs. Through repeated trituration and firing all the surface and core area are comes in contact of heat sufficiently leading to proper reaction process and desired compound was obtained [6].
After etching, the outer sections of the grain boundaries with the etched surface are marked by a network of shallow cliffs. These nearly vertically surfaces do not reflect the light into the objective lens of a microscope and as a result a dark area is observed. And bright area was observed when light reflects from the plane surface and passes into the objective lens of the metallographic microscope [7].
In bhasma the size of the grain was significantly reduced and possibly many compounds other than the raw material is formed that may be due to the process effect.
Conclusion
Metallographic analysis shows the conchoidal structure of the raw SM
Shodhita SM shows the Breaking of the structure of raw material, formation of some other compounds over the surface of the grain and appearance of some free metal in the sample
The grain size of the Bhasma significantly reduced, the orientations of the grains were also changed and the grains of more oxide compounds were found with some sulphide compound
The data of the metallographic studies of the raw SM and bhasma may be the fingerprint for the same manufacturing process followed.
Glossary
Shodhana
Traditional process, where raw materials are purified, and inducted with therapeutic property. It is a pre -process for marana
Marana
It is a traditional process done for the preparation of bhasma by using different associated materials and providing proper amount of heat.
Bhavana
Ancient process of tritutration done in wet condition by adding certain liquid media
Puta
It is the quantum of heat required to achieve desired product from a specified raw material.
Nischandra:
A quality control tests for bhasma meaning loss of any shinning or lustureness.
Amlaparikshya
Prepared bhasma is mixed with lemon juice or curd and observed for any color change. No color change confirms the well prepared bhasma.
Varitara
Some bhasma particle is sprinkled over water and observed; if it floats bhasma is well prepared and on sinking it is rejected. It claims lightness of the bhasma.
Rekhapurna
The bhasma particle enters into the cleavage of the linings of the finger. It signifies the fineness of the bhasma.
References
- Sharma Sadananda, Rasa Tarangini,11th Edition, Reprint 2004, Motolal Banarasidas Publication, Varanasi.
- Acharya Bagbhatta, Rasa Ratna Samucchhaya, edited by D.A Kulkarni, 1969, ML publication, New Delhi.
- Sharma Sadananda, Rasa Tarangini,11th Edition, Reprint 2004, Motolal Banarasidas Publication, Varanasi.
- George L Kehi, The Principles of Metallographic laboratory practice, Published by Eurasia Publishing House, Pvt. Ltd, New Delhi, 3rd Edition 1965.
- Bhabesh Das et al, Metallographic Study of Metallic Bhasmas, Dept. of Rasa Shastra, IMS, BHU, Varanasi, 1989
- Mohapatra et al, Process Standardization of Swarna Makshika bhasma and it experimental evaluation for hypnotic and behavioral effect on experimental animal, Dept. of Rasa Shastra, IMS, BHU, 2006
- B. Jha et al, A Study on Satvapatana of Abhraka and Makshika, Department of Rasa Shastra, IMS, BHU, Varanasi, 1990.
- A.K.Choudhary et al, Studies on bhasmas of Makshika and Makshika satva, Department of Rasa Shastra, IMS, BHU, Varanasi, 1997.
- Durga Chinta et al, Comparative study of Makshika bhasma and the bhasma prepared With the combination of Tamra & Loha, Dept of Rasa Shastra, IMS, BHU, 2005







