Manuscript accepted on :October 25, 2009
Published online on: 17-11-2015
Plagiarism Check: Yes
Omair Hasan Osmani¹*, Dhanasekar Sathish Sekar¹, K.L. Senthil Kumar¹, Ram Kumar Sahu² and Amit Roy³
¹Department of Pharmacognosy, Padmavathi College of Pharmacy, Dharmapuri India.
²Department of Pharmacognosy, Oriental College of Pharmacy, Bhopal India.
³Columbia College of Pharmacy, Mandhar, Raipur India.
Corresponding Author E-mail:b_omair2006@yahoo.co.in
Abstract
Artocarpus heterophyllus is a medicinal plant, used by herbalist for treating various diseases, one of which is Diabetes mellitus. The aim of this study was to investigate the antidiabetic potential of Artocarpus heterophyllus plant seed powder. This was tested in normal and strepozotocin induced diabetic rats using oral administration of ethanol extracts of Artocarpus heterophyllus. After the oral administration of ethanol extract at doses of 400 mg/kg body weight, blood glucose levels were monitored at specific intervals and found significantly lowered the blood glucose level. Glibenclamide was used as a standard drug at a dose of 0.25 mg/kg. The experimental data revealed that extract has significant antihyperglycaemic activity in streptozotocin-induced rats compared to standard drug. The extracts seem promising for the development of an oral antidiabetic medicine.
Keywords
Antidiabetic; Lipid profile; Artocarpus heterophyllus; Strepozotocin; Glibenclamide
Download this article as:| Copy the following to cite this article: Osmani O. H, Sekar D. S, Kumar K. L. S, Sahu R. K, Roy A. In Vivo Antidiabetic Potential of Artocarpus Heterophyllus Plant Seeds in Streptozotocin-Induced-Diabetic Rats. Biomed Pharmacol J 2009;2(2) |
| Copy the following to cite this URL: Osmani O. H, Sekar D. S, Kumar K. L. S, Sahu R. K, Roy A. In Vivo Antidiabetic Potential of Artocarpus Heterophyllus Plant Seeds in Streptozotocin-Induced-Diabetic Rats. Biomed Pharmacol J 2009;2(2).Available from: http://biomedpharmajournal.org/?p=825 |
Introduction
Every year the number of diabetic patients is growing alarmingly all over the World. Diabetes is a chronic disease characterized by derangement in carbohydrate, fat, protein metabolism. Most of the hypoglycemic agents used in allopathic medicines are reported to have side effects in the long run. Therefore, there is a need to search for effective and safe drugs for diabetes.
The jackfruit (Artocarpus heterophyllus) is a species of tree in the mulberry family (Moraceae), which is native to parts of South and Southeast Asia. It is called Katahal in Hindi, medium-size evergreen tree typically reaching 8–25 m (26–82 ft) in height that is easily recognized by its fruit. Its fruit is the largest tree borne fruit in the world, seldom less than about 25 cm in diameter. The jackfruit is something of an acquired taste, but it is very popular in many parts of the world. Seeds are light brown to brown, rounded, 2–3 cm in length by 1–1.5 cm in diameter, and enclosed in a thin, whitish membrane and has a sweet taste. Up to 500 seeds can be found in each fruit The nutritious seeds are boiled or roasted and eaten like chestnuts, added to flour for baking, or cooked in dishes (1-4). The main objectives of this study was to assess the antidiabetic potential of ethanol extracts of powdered seeds of Artocarpus heterophyllus in control of blood glucose levels and effectiveness on various biochemical parameters viz total cholesterol, triglycerides (TGL), high density lipoprotein, (HDL), low density lipoprotein, (LDL), very low density lipoprotein, (VLDL).
Materials and Methods
Plant material
The fruit seeds Artocarpus heterophyllus has been collected from Hyderabad district of Andhra Pradesh, India. The species for the proposed study was identified as Artocarpus heterophyllus by Dr. P. Jayaraman, Botanist, Plant Anatomy Research Centre (PARC), Chennai.
Preparation of Artocarpus heterophyllus seeds ethanolic extract
The powder of seeds (300gm) of Artocarpus heterophyllus, was packed well in Soxhlet apparatus and extracted with ethanol until the completion of the extraction. The extract was filtered while hot, and the resultant extract was distilled in vacuum under reduced pressure in order to remove the solvent completely and dried in a desiccator (5).
Animals
Male wistar albino rats having weight 170-220gm were kept in quarantine for 10 days under standard husbandry conditions (27.3o, Relative humidity 65 ±10%) for 12 hrs in dark and light cycle respectively and were given standard food and water ad. libitum. The study was permitted by the Institution Animal Ethical Committee.
Acute toxicity testing
Acute toxicity testing was performed for ethanol extract following OECD guidelines-420, fixed dose Procedure. Where a fixed dose level of extract starting from 50, 100, 200, 500, 1000, increasing upto 2000mg/kg body weight was given, sign and symptoms of toxicity were observed for next 48 hrs. No toxicity or death was observed in experimental rats (6, 7).
Oral glucose tolerance test (OGTT)
The oral glucose tolerance test (8) was performed in overnight fasted (18 h) normal rats. Rats divided into two groups (n = 6) were administered drinking water, Artocarpus heterophyllus ethanol extract (400 mg/kg), respectively. Glucose (2 g/kg) was fed 30 min prior to the administration of extracts. Blood was withdrawn from the retro orbital sinus at 30, 60 and 120 min of extract administration and the plasma obtained after centrifugation at 3000 rpm was estimated for fasting plasma glucose levels using a glucose oxidase–peroxidase glucose estimation kit.
Induction of non-insulin dependent diabetes mellitus (NIDDM)
NIDDM was induced (9,10) in overnight fasted adult Wistar strain albino male rats weighing 170–220 gm by a single intraperitoneal injection of 60 mg/kg streptozotocin , 15 min after the i.p. administration of 120 mg/kg of nicotinamide. Streptozotocin (STZ) was dissolved in citrate buffer (pH 4.5) and nicotinamide was dissolved in normal saline. Hyperglycemia was confirmed by the elevated glucose levels in plasma, determined at 72 h and then on day 7 after injection. The threshold value of fasting plasma glucose to diagnose diabetes was taken as > 126 mg/dl. Only those rats were found to have permanent NIDDM were used for the study.
Experimental design
The animals were segregated into four groups of six rats each. The extract was administered for 12 days. Group I served as normal control rats administered drinking water daily for 12 days; Group II diabetic control rats administered drinking water daily for 12 days; Group III diabetic rats administered ethanol extract (400 mg/kg); Group V diabetic rats administered standard drug Glibenclamide (0.25 mg/kg) for 12 days. The fasting glucose levels were determined on day 1, 5 and 12 of extract administration.
Estimation of biochemical parameters
The biochemical parameters were determined on day 12 after the animals were sacrificed by cervical dislocation. Total cholesterol, triglycerides (TGL), high-density lipoprotein (HDL), low-density lipoprotein (LDL), very low-density lipoprotein (VLDL), by Glucose oxidase method using auto-analyzer (11, 12).
Histopathology
All the animals were sacrificed on 12th day by cervical dislocation, pancreas were isolated and were subjected to histopathological studies and microscopical findings were noted.
Statistical Analysis
The results are expressed as mean ± SEM of six independent experiments. Statistical significance between group was evaluated by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. A P < 0.05 value was considered as statistically significant.
Results And Discussion
In our study, the difference observed between the initial and final fasting plasma glucose levels of different groups under investigation revealed a significant elevation in blood glucose in diabetic control group as compared with normal animals at the end of the 12-day experimental period. When ethanol extract of Artocarpus heterophyllus was administered to glucose loaded normal rats fasted for 18 h, significant decrease in plasma glucose level was observed after 30 min. The extracts reduced plasma glucose level to normal at 90 min (Table 1). During study it was found that extract control significantly the blood glucose level on streptozotocin induced diabetic rats. The ethanol extract induced a significant reduction on blood glucose level in STZ-induced-diabetic rats as compared to the diabetic control group (Table 2). But ethanol extract showed significant antidiabetic activity as compared to standard drug. The possible mechanism by which Artocarpus heterophyllus brings about its hypoglycemic action in diabetic rat may be by potentiating the insulin effect of plasma by increasing either the pancreatic secretion of insulin from the existing beta cells or by its release from the bound form.
Table 1: Effect of ethanol extract of Artocarpus heterophyllus on oral glucose tolerance test.
| Group | Plasma glucose concentration (mg/dl) | ||
| 30 min | 60 min | 90 min | |
| Normal control | 72.55±1.94 | 97.79±2.07
|
95.49±2.17 |
| Normal + Ethanol Extract | 66.76±1.76 | 83.88±2.33* | 72.87±1.27* |
Values are expressed as mean ± SEM (Number of animals, n=6); * Significantly different from the normal control at P<0.05
Table 2: Effect of ethanol extract of Artocarpus heterophyllus on fasting plasma glucose level in rats.
| Group | Fasting plasma glucose concentration (mg/dl) | ||
| Day 1 | Day 5 | Day 12 | |
| Normal control | 82.21±1.43 | 84.87±1.87 | 81.48±1.83 |
| Diabetic control (Streptozotocin) | 199.4±1.62* | 212±2.29* | 236.6±2.79* |
| Diabetic + Ethanol Extract(400 mg/kg) | 196.6±1.86* | 122.6±2.91* | 94.15±2.85* |
| Diabetic + Standard Glibenclamide (0.25 mg/kg) | 200±2.69* | 102.9±2.29* | 80.90±1.83 |
Values are expressed as mean ± SEM (Number of animals, n=6); * Significantly different from the normal control at P<0.05.
Abnormalities in lipoproteins are very common in both NIDDM and IDDM. Although lipoprotein alterations appear to be an intrinsic part of these disorders, such alterations are also induced by diabetes associated complications such as obesity and renal disease (13). The total cholesterol, triglyceride levels, VLDL and LDL were observed to be elevated in diabetics but reduced by extract significantly as well as glibenclamide showing their beneficial effects. In the present study, HDL levels remained unchanged in diabetics compared to the other groups (Table 3). These results suggest the beneficial effects of the natural extract in improving the imbalance in lipoprotein metabolism are also comparable to those of glibenclamide.
Table 3: Determination of biochemical parameters after treatment with ethanol extract of Artocarpus heterophyllus and Glibenclamide.
| Group | Total cholesterol (mg/dl)
|
HDL (mg/dl)
|
Triglycerides (mg/dl)
|
VLDL
|
LDL
|
| Normal control | 84.20±3.01 | 54.41±2.69 | 85.35±2.91 | 34.4±2.69 | 43.56±3.64 |
| Diabetic control (Streptozotocin) | 154.43±4.04* | 51.13±1.97 | 182.5±3.08* | 68.7±3.51* | 160.3±4.43* |
| Diabetic + Ethanol Extract(400 mg/kg) | 74.8±2.84 | 46.87±3.34 | 103.5±3.46* | 25.02±3.45 | 82.84±2.99* |
| Diabetic + Standard Glibenclamide (0.25 mg/kg) | 60.75±4.32* | 48.6±3.15 | 90.99±4.52 | 31.54±1.92 | 38.52±2.42 |
Values are expressed as mean ± SEM (Number of animals, n=6); * Significantly different from the normal control at P<0.05
The histopathological study of normal group (Fig. 1a) showed a normal pancreatic structure. The pancreas presents in the group of animals treated with ethanol extract (Fig. 1c) showed more or less similar appearance of pancreatic lobules, acing and cells as compared to standard drugs (Fig. 1d), whereas pancreas of diabetic control animals showed partially damaged or even destroyed pancreatic lobules, acing, cells, islet size, shaped and number (Fig. 1b).
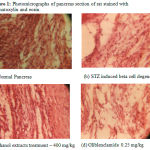 |
Figure 1: Photomicrographs of pancreas section of rat stained with haematoxylin and eosin.
|
Ethanol extract (400mg/kg) showed significant antidiabetic activity when compared with standard drug (Glibenclamide 2.5 mg/kg). Present work have indicated the fact that the plant Artocarpus heterophyllus, has antidiabetic constituents and production of a safe antidiabetic drug is very much possible from the seeds.
References
- Oriental Longmann, Indian Medicinal Plants, Vol. I, Oriental Longman Ltd., 51-54 (1999).
- Chandrika, U.G., Wedage, W.S., Wickramasinghe, S.M.D.N., and Fernando, W.S., “hypoglycaemic action of the flavonoid fraction of Artocarpus heterophyllus leaf” Afr. J. Trad. CAM, 3 (2); 42-50 (2006).
- Craig, R.E. and Harley, I.M., “Artocarpus heterophyllus (jackfruit)” Species Profiles for Pacific Island Agroforestry, 4; 1-16 (2006).
- Bramhaverchas, Ayurveda Ka Pran: Vanoausdhi Vigyan, Vol. III, Vedmata Gyatri Trust, Haridwar, 80-81 (2003)
- Sharma, S.B., Nasir, A., Prabhu, K.M., Murthy, P.S. Dev, G., “Hypoglycemic and hypolipidemic effect of ethonolic extract of seed of Eugenia jambolana in alloxan-induce diabetic rabbits” Journal of Ethanopharmacology, 85 (2-3); 201-206 (2003).
- Ecobichon, D.J., “Fixed Dose Procedure Guidline 420” The Basis of Toxicity Testing, 2nd edition, CRC Press, 1997.
- Ghosh, M.N. In: Fundamentals of Experimental Pharmacology, Scientific Book Agency, Calcutta, 1984.
- Shriwaikar, A., Rajendran, K., Punitha, I.S., “Antidiabetic activity of alcoholic stem extract of Coscinium fenestratum in Streptozotocin-nicotinamide type 2 diabetic rats” Journal of Ethnopharmacology, 97; 369-374 (2005).
- Angel, I., Burcelin, R., Girard, J. , Salomon, Z., “Normalization of insulin secretion by selective alpha-2 adrenoceptor antagonist receptors GLUT-4 glucose transporter expression in adipose tissue of type- II diabetic rats” Endocrinology, 137; 2022–2027 (1996).
- Pellegrino, M., Christophe, B., Rene, G., “Development of a new model of type II diabetes in adult rats administered with Streptozotocin and nicotinamide” Diabetes, 47; 224 (1998).
- Nyarko, A.K., Sittie, A.A., Addy, M.E., “The basis for the antihyperglycaemic activity of Indigofera arrecta in the rat” Phytotherapy Research, 7; 1–4 (1993).
- Barham, D., Trinder, P., “An improved color reagent for the determination of blood glucose by the oxidase system” Analyst, 97; 142-145 (1972).
- Virdi, J., Sivakami, S., Shahani, S., Suthar, A.C., Banavalikar, M.M., Biyani, M.K., “Antihyperglycemic effects of three extracts from Momordica charantia” Journal of Ethanopharmacology 88; 107-111 (2003).







