Manuscript accepted on :July 05, 2009
Published online on: 18-11-2015
Plagiarism Check: Yes
V. P. Pandey1 and J. K. Pandit2
1Department of Pharmacy, Annamalai University, Annamalainagar - 608 002 (India). 2Department of Pharmaceutics, I. T., B.H.U., Varansi - 5 India.
Abstract
A bioavailability study o one commercial and four fabricated tablet formulations of sulfisoxazole was conducted in four (for one formulation three subjects) healthy human volunteers with single oral 1g dose. The possible effect of additives on the urinary excretion pattern of sulfisoxazole was examined. The drug was analysed in urine for ‘total’ and ‘free’ forms. Although two fabricated formulation gave significantly lower extent of bioavailability, the other two fabricated tablets were not significantly different from the reference commercial product. The slow rate of absorption for one formulation was observed to be associated with long dissolution time.
Keywords
Sulfisoxazole; bioavailability; tablets; additives
Download this article as:| Copy the following to cite this article: Pandey V. P, Pandit J. K. In Vitro and In Vivo Evaluation of some Tablet Formulations of Sulfisoxazole. Biomed Pharmacol J 2009;2(2) |
| Copy the following to cite this URL: Pandey V. P, Pandit J. K. In Vitro and In Vivo Evaluation of some Tablet Formulations of Sulfisoxazole. Biomed Pharmacol J 2009;2(2).Available from: http://biomedpharmajournal.org/?p=876 |
Introduction
Sulfisoxazole in usually administered by mouth and prescribed in the treatment of infections of the urinary tract. By virtue of the large number of diverse types of excipients needed in their manufacture, tablets are potentially proved to show variations in biological availability. Various parameters such as particle size, crystal form, aqueous solubility, wettability of the drug and excipients like diluents, binders, lubricants, glidants and granulating agents influence drug bioavailability from tablets. In recognition of the potential low bioavailability of some drugs from tablets, the USP XXII 1990 has prescribed dissolution minimum. Sulfisoxazole is one such drug for which dissolution test in prescribed.
In view of the USP XXII 1990 specification for dissolution test, the present work was undertaken to evaluate the performance of a commercial tablet (M) of sulfisoxazole in comparison to four fabricated tablet dosage forms (C1, C2, S1 and S2), two of which were of the fast release type (Chewable, C1 and C2) and two were normal swallow tablet formulations (S1 and S2). in vitro and in vivo studies were performed. The present study describes the excretion pattern of the drug from the different tablet formulations.
Material
Sulfisoxazole, mannitol, lactose, polyethylene glycol 4000 (PEG 4000), talc, starch, magnesium stearate, sucrose, polyvinyl pyrrolidone (PVP), citric acid, fumed silica (cab-o-sil), and all other ingredients were of either USP or analytical reagent grade and procured from commercial sources. They were used as received.
Methods
Tablets were prepared by standard methods. Various types of ingredients used and their quantity are described in Table 1. The granulation with PVP was done for chewable tablet formulation C1 (Chalmers and Elworthy, P.H. 1976; Chalmere and Elworthy, P.G. 1976). In all cases dried granules were passed through 20 mesh sieve and the portion retained above 40 mesh was separated. PEG 4000 dissolved in small volume of ethanol was sprayed evenly over the granules of tablet formulations C2 and S2. Prior to compression, lime flavour dissolved in a small volume of ethanol was incorporated in the granules of formulations C1 and C2. The wet granulation technique was employed in the preparation of S1 fabricated tablet formulation. Compression was done in a Manesty E2 type single punch machine, for all the four fabricated formulations. 3/8 inch that die punch set was used for compression of S1, C1, C2 and S2 formulations were compressed using half inch flat die punch set.
The hardness of C2 was deliberately kept around 5.0 kg/cm2 lower than hardness around 7.0 kg/cm2 of C1, S1 and S2.
Disintegration time and weight variation percent were found out as per USP XXII specifications using USP standard apparatus. Hardness and friability were measured by Monsanto hardness tester and Roche friabilator.
Four healthy human subjects (weight 55-60), of whom none were smoker completed the study for formulations M, C1, C2 and S2/. One subject dropped out during the study of formulation S1 for his personal reasons. All subjects were drug free for at least two weeks prior to and until completion of the study. They were also asked to refrain from alcoholic and caffeinated beverages 48 hours (h) prior to each dosing and until 24 h after the collection of the last urine sample.
After an over-night fast, each subject ingested in 1g dosing of sulfisoxazole of the same type and washed down with 240 ml of water with adequate cross over. Standardized meals were supplied at 4, 8 and 12 h of each administration. Urine samples were collected at 1, 2, 3, 5, 7, 9 and 24 h after dosing. Urine samples voided beyond 9 h and upto 24 h were pooled. Volume of urine samples was measured and a 10 ml aliquot was collected in evacuated glass tubes with car being taken to avoid contact of urine with rubber stoppers.
The urine concentrations of ‘free’ and ‘total’ sulfisoxazole were quantitated by Bratton and Marshall method (1939). The hydrolysis of conjugate, N4-acetyl sulfisoxazole, was done by adding 1 ml of 10 M Hcl to 1 ml urine sample and operating this mixture on water bath for 1 h at 98oC. Thus, two levels of the drug, i.e., ‘free’ sulfisoxazole and ‘total’ sulfisoxazole were measured for each urine sample. The test subjects voided urine just before the drug intake, which served as the 0 hours or blank sample in the analysis procedure. The pH of all urine samples were recorded to keep a check. To maintain an adequate urine output, the volunteers were advised to drink 240 ml of water after each voiding of urine.
Results and Discussion
The present study was undertaken to investigate the effect of some formulation additives on the urinary excretion pattern of sulfisoxazole being one of the drugs possessing extremely low aqueous solubility. The USP XXII has specified a dissolution minimum of not less than 70% of the drug dissolved in 30 minutes in 0.1 N HCl. Accordingly, the formulation was divided into two types contained excipients which have either high affinity for aqueous fluids, or are highly water soluble (Table 1).
The commercial tablet was chosen as the standard preparation in the in vivo study as it showed excellent in vitro characteristics in preliminarily studies (Table 2). Dissolution test results are shown in Fig. 1. Commercial tablet formulation M exceeded the USP XXII dissolution specification; in that almost 94.1% of the drug had dissolved in 30 minutes, whereas the amounts dissolved form fabricated tablet formulation S1 and S2 in the same time are 70.5% and 90.1% respectively. Although S1 is having a lower hardness than the commercial tablet formulation M, but the disintegration time of the latter is almost equal to that of S1. Hence, hardness is not a factor controlling either disintegration time or dissolution rate in this case (Table 2, Fig. 1).
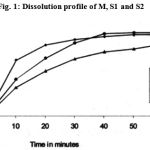 |
Figure 1: Dissolution profile of M, S1 and S2.
|
Again, the slightly higher hardness of S2 results in an enormously increased disintegration time, though the disintegration time is within the pharmacopoeial limits. The reason for this sudden jump in disintegration time with a very small increase in hardness is probably brought about by the shape factor. The M was larger in diameter and thinner than the S2 which seems to have brought about quicker disintegration of tablet formulation M. Table 2 show that S1 is having poor dissolution result in spite of its low hardness and small disintegration time.
It is found that tablet formulation S2, with a higher disintegration time and hardness, is comparable to commercial product M is its dissolution characteristics. It is probable that starch paste, used as a granulating agent in the formulation of S1, produced heavy binding of the drug particles, as a result of which the release of individual drug particles was slowed down and consequently the dissolution rate was also slow. This is in spite of the fact that S1 showed a much lower disintegration time than S2. This apparent discrepancy may be explained on the basis that the disintegrant, starch, present in S1 brought about early bursting of the tablets and quick release of granules, further breakdown of individual granules into primary drug particles was not forthcoming. Contrary to this, S2 had a higher hardness and disintegration, but the highly water soluble sucrose although gave hard tablets, as is its usual characteristics, it (sucrose) dissolved away from the intra granular irregularities at a fast rate and released primary drug particles much earlier. So it is proposed that the choices of a granulating and binding agents are to be made very judiciously in the formulation of sulfisoxazole tablets and in doing so disintegration time may not be considered as the sole guiding characteristic of a good tablet formulation, Rather, a complete dissolution test run on the lines of direction of the USP XXII would be more appropriate.
The results show that all five products met specifications for assay and content uniformity of USP XXII 1990 (Table 2).
Sulfisoxazole was well tolerated by the subjects. All the subjects completed the study without any complaint and complication.
The performance of the in vivo study of the tablet formulations are shown in Table 3 it is observed that although the inter subject variation in drug excretion is large, as is commonly found in human volunteer studies, the mean cumulative excretion of both the free drug and the metabolite (free deducted from total) are mostly similar except for fabricated swallow tablet formulations S1. Even for S1 the values are significantly different only from 5 h onwards post administration. Indeed the excretion of metabolite from this tablet formulation S1 is also not significantly different from the commercial tablet M between the second and the ninth h (values not reported).
Sulfisoxazole is a drug with a low renal extraction ratio. In this case the clearance of the drug will depend largely on the enzymatic systems metabolizing the drug. Since the half – life (t1/2) is an indication of the efficiency of the elimination processes of the body, any change in the half-life will reflect changes in these elimination organ functions. In the present study it was found that although the absorption rates of sulfisoxazole from fabricated tablet dosage forms different significantly from the commercial product, the average half lives and elimination rate constants were similar
(Table 3).
From the Table 3, the elimination rate constants (k) are not much different from on product to another but absorption rate constants (ka) are significantly lower in case of C1, C2 and S1 than the M and S2. The absorption rate constants values (Ka) for M and S2 are almost similar. These values are not unexpected, because the dissolution rate of S2 is very close to that of M (Fig.1). In the present study it was found that the rate of urinary excretion of formulation M was more close to formulation S2 (values not reported).
Results of in vivo and in vitro studies show that the commercial tablet formulation M is superior to self fabricated tablet formulations C1, C2, S1 and S2. The following factors may be responsible separately or in combinations(s), (a) particle size of drug particle (b) excipients and (c) manufacture technique.
The performance of fabricated tablet formulations can be described in the order S2>C1>C2>S1 (Table 3). It may be proposed that the excipients, sucrose, bears larger responsibility for the results of S2 and C1.
From Table 3, relative bioavailability showed that neither dissolution minima (S1) nor mastication (C2) may be useful for being bioequivalent. It is suggested that complete study on bioavailability should be performed and it should be made mandatory. Thus the present study gives an excellent description of the effect of excipients used in tablet formulations on the urinary excretion pattern of sulfisoxazole.
Limited number of human subjects were selected fro this study because of practical problems faced in urine sampling and frequent drop out of subjects on their personal grounds. The study was conducted in 1983-84.
References
- Bratton, A. C. and E. K. Marshall, Jr. A New Coupling Component for Sulfanilamide Determination, J. Bio. Chem., 120: 537-550 (1939).
- Chalmers, A. A. and Elworthy, P. H. Effect of Variations in Binder Concentration and Volume on Granule and Tablet Properties, J. Pharm. Pharmacol. 28(3): 228-233 (1976).
- Chalmers, A.A. and Elworthy, P. G. Oxytetracycline Tablet Formulation: The Effect of Wet Mixing Time, Particle Size and Batch Variation on Granule and Tablet Properties, J. Pharm. Pharmacol., 28(3): 239-243 (1976).







