Manuscript accepted on :September 15, 2009
Published online on: 18-11-2015
Plagiarism Check: Yes
S. Padmapriya*, J. Kausalya, V. Vaijayanthi, S. K. Umadevi, Deepak Patil and B. Senthilnathan
Vels College of Pharmacy, Pallavaram, Chennai- 117 India.
Abstract
The present study was to develop sustained release matrix tablet of Losartan potassium a potent first angiotensin antagonist, using hydrophilic and hydrophobic polymers (HPMC, SCMC and EC) alone and in combination in the ratio 1:1. Preformulation studies were carried out to rule out interaction between drug and polymer. The granules (six formulations) were prepared by wet granulation method and evaluated for Bulk Density, Angle of repose, CI, Total porosity and drug content. The granules showed satisfactory flow properties, compressibility and drug content. The granules were then compressed into tablets and subjected to thickness, weight variation, hardness, drug content and in vitro release studies. All the tablet formulation showed acceptable pharmacotechnical properties and compliance with in-house specifications for tested parameters. The result of vitro release study revealed that formulation containing hydrophilic and hydrophobic polymers combination showed sustained release characters with diffusion coupled erosion mechanism.
Keywords
Sustained release; matrix tablet; Losartan potassium; hydrophilic; hydrophobic polymers
Download this article as:| Copy the following to cite this article: Padmapriya S, Kausalya J, Vaijayanthi V, Umadevi S. K, Patil D, Senthilnathan B. Development and Evaluation of Iosartan Potassium Sustained Release Matrix Tablet using Hydrophilic and Hydrophobic Polymers. Biomed Pharmacol J 2009;2(2) |
| Copy the following to cite this URL: Padmapriya S, Kausalya J, Vaijayanthi V, Umadevi S. K, Patil D, Senthilnathan B. Development and Evaluation of Iosartan Potassium Sustained Release Matrix Tablet using Hydrophilic and Hydrophobic Polymers. Biomed Pharmacol J 2009;2(2).Available from: http://biomedpharmajournal.org/?p=891 |
Introduction
Losartan potassium is a potent, highly specific angiotensin II type 1 (AT1) receptor antagonist with anti-hypertensive activity1. It is readily absorbed from the GIT with oral bioavailability 33%, plasma elimination half life ranging from 1.5 – 2.5h and freely soluble in water2 hence it is considered as a model drug for development of SRDDS. The most commonly used method of sustaining drug release is matrix system. Hydrophilic polymer matrix systems are widely used for designing SRDDS because of their flexibility to provide a desirable drug release profile, cost effectiveness and broad regulatory acceptance3-5. HPMC has been used most frequently in the formulation of sustained release monolithic matrix tablet because of its hydrophilic gel-forming property, non-toxicity and cost effectiveness6-8. However, the use of hydrophilic matrix alone for sustaining drug release for highly water soluble drugs is restricted due to rapid diffusion of the dissolved drug through the hydrophilic gel network. For such drug it becomes essential to include hydrophobic polymers in the matrix system9-13. Hence in the present work, an attempt has been made to formulate sustained release matrix tablet of Losartan potassium using hydrophilic (HPMC, SCMC) in combination with hydrophobic (Ethyl cellulose) polymers.
Materials and Methods
Materials
Losartan potassium was provided ex gratia by Wockhard Labs (Aurangabad, India). HPMC and Ethyl cellulose were supplied by Rankem limited (India), Sodium CMC provided by LOBA chemie (India), magnesium stearate and talc were purchased from S.D. Fine Chemicals (India).
Preparation of compressed matrices
Losartan potassium SR tablets were prepared by wet granulation method14. Drug and the lactose (diluents) were sifted through #40 manually and mixed well to ensure the uniformity of premix blend. Several drug-diluents premixes were then mixed with the selected combination and ratio of hydrophilic and hydrophobic polymers (HPMC, SCMC, Ethyl cellulose), previously sifted through #40, for 5 min. Premix blend was wet granulated with starch paste and the granules were sized through #18 and were dried at 45 oC for 15 min. Dried LP granules were lubricated with talc and magnesium stearate and compressed into tablets.
Six formulations of Losartan potassium 100 mg sustained release tablets were prepared using following excipients: HPMC (50-100 mg), SCMC (50-100 mg), Ethyl cellulose (50-100mg) talc (10.25 mg), magnesium stearate (1.75 mg) and lactose (q.s. to 300 mg).
Characterization of granules
The granules of all six formulations were subjected to characterization studies namely LBD, TBD, Hausner ratio, Angle of repose, Compressibility Index.15
Characterization of tablets
The tablets of all six formulations were subjected to various evaluation tests such as thickness, weight variation, hardness, friability and drug content16.
In vitro drug release studies
Dissolution studies were performed using the USP XXVIII, paddle-rotating method (Electrolab dissolution tester, Electrolab, India) at 37 _C ± 0.5 _C and 75 rpm using 0.1 N HCl (2 h) and phosphate buffered solution, pH 6.8 (PBS) (10 h), as the dissolution media. Dissolution studies were carried out in triplicate, maintaining the sink conditions for all the formulations. 5 ml aliquot of sample was withdrawn at regular time intervals, filtered and assayed spectrophotometrically at 205 nm. The cumulative % drug release was calculated for the formulations17.
Mechanism of drug release
To study the release mechanism, various dissolution models were applied to the in vitro release profiles of the six formulations. The kinetic models includes zero order, first order, higuchi’s and korsmeyer model9.
Result and Disscussion
The result of LBD and TBD of all the formulation ranged from 0.321±0.012 to 0.363±0.01g/ml and 0.382±0.012 to 0.412± 0.0135 g/ml. respectively Bulk Density of granules prepared using Ethyl cellulose combination was found to be quite higher than those of other granules due to hydrophobic nature. The results of angle of repose (<30) for all formulation indicate good flow properties. This was further supported by lower compressibility index values. The drug content in the weighed amount of granules of all formulations was ranged from 97.61 to 99.21% found to be uniform. Thus all these results indicate that the granules possessed satisfactory flow properties, compressibility, and drug content. All formulation showed uniform thickness in the range 3.04±0.01 to 3.45±0.04mm and tablet weights varied between 297.3-298.6mg. Hardness, friability and drug content of all formulation were in the range 5.5-6.5kg/cm2, 0.53-0.37% and 97.42-99.0% respectively. Thus all the tablets showed acceptable pharmacotechnical properties and complied with the in-house specifications for weight variation, hardness, friability and drug content (Table-I).
Table 1:Characterization of granules and matrix tablets of Losartan potassium*
| Parameters | F1 | F2 | F3 | F4 | F5 | F6 |
| Granules
LBD (g/cm3) TBD (g/cm3) CI (%) Angle of repose(o) Total porosity (%) Drug content (%) |
0.33± 0.01 0.39 ±0.01 14.5±0.01 25.9±0.01 29.5±0.03 98.0±0.01 |
0.36±0.01 0.41±0.01 13.96±0.01 25.98±0.01 27.2±0.01 97.6±0.01 |
0.34±0.01 0.38±0.01 15.3±0.02 28.3±0.03 28.2±0.01 99.2±0.01 |
0.41±0.01 0.41±0.01 15.3±0.01 25.8±0.02 29.5±0.02 98.4±0.01 |
0.36± 0.01 0.45±0.01 13.4±0.01 25.4±0.02 26.5±0.01 98.9±0.01 |
0.32 ±0.01 0.38±0.01 15±0.02 25.8±0.01 27.3±0.01 97.9±0.01 |
| Tablets
Thickness (mm) Weight variation (%) Friability (%) Hardness (kg/cm2) Drug content |
3.04±0.01 ±2 0.5±0.01 5.5±0.05 98.1±0.01 |
3.36±0.01 ±3 0.4±0.01 6.1±0.1 97.5±0.02 |
3.45±0.01 ±4 0.4±0.01 5±0.05 98.5±0.01 |
3.43±0.01 ±4 0.3±0.01 6.3±0.1 97.4±0.01 |
3.05±0.01 ±3 0.5±0.01 6±0.5 98.9±0.01 |
3.25±0.01 ±3 0.5±0.01 5.5±0.1 98±0.01 |
Mean ± SD (n=3)
In vitro dissolution study
The results of dissolution studies indicated that formulation (Losartan potassium with SCMC with Ethyl cellulose) produced sustained effect with 74.19% of drug release over a period of 12hr. in comparison to other formulation and diffusion coupled with erosion suggested mechanism (Figure I).
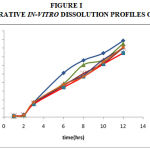 |
Figure 1: Comparative In-Vitro Dissolution Profiles Of F1- F6.
|
To know the mechanism of drug release from these formulations, the data were treated according to zero order (cumulative amount of drug released vs time), first-order (log cumulative percentage of drug remaining vs time), Higuchi’s (cumulative percentage of drug released vs square root of time), and Korsmeyer (log cumulative percentage of drug released vs log time) equations along with pattern. The results of release rate kinetic data for all the other equations can be seen in Table II.
Table 2: Release Kinetic Study.
| Formulation | Zero order | First order | Higuchi’s equation | Korsmeyer |
| F 1 | 0.974 | 0.827 | 0.993 | 0.505 |
| F 2 | 0.979 | 0.836 | 0.997 | 0.711 |
| F 3 | 0.964 | 0.833 | 0.994 | 0.669 |
| F 4 | 0.980 | 0.832 | 0.997 | 0.443 |
| F 5 | 0.974 | 0.812 | 0.996 | 0.645 |
| F 6 | 0.966 | 0.813 | 0.993 | 0.609 |
In our experiments, the in vitro release profiles of drug from the entire formulations studied using kinetics model, when the data were plotted according to the zero-order equation, the formulations showed a good linearity, with regression values between 0.966 and 0.980. It could be best expressed by Higuchi’s equation, as the plots showed high linearity (R2: 0.997 to 0.993). To confirm the diffusion mechanism, the data were fit into Korsmeyer equation. The formulations F-1 to F-6 showed with slope (n) values ranging from 0.711 to 0.443, indicating that diffusion is the dominant mechanism of drug release with these formulations. When plotted according to Korsmeyer equation, formulation F-5 also showed with a slope (n) value of 0.645. This n value, however, appears to indicate a coupling of diffusion and erosion mechanisms so called anomalous diffusion.
Summary
Losartan potassium is one of the first orally active angiotensin II antagonist used in the treatment of hypertension either alone or in combination with hydrochlorothiazide. The objective of the present is to investing the possibility of sustaining of Losartan potassium release from matrix tablet prepared by hydrophilic and hydrophobic polymer.
The preformulation studies were carried out which ruled out the interaction between the drug and polymers. Losartan potassium granules (six formulations) were prepared by using wet granulation method using hydrophilic and hydrophobic polymers namely HPMC, SCMC and EC respectively alone and combination in the ratio 1:1. The granules were evaluated for LBD, TBD, compressibility Index, Hausner ratio, angle of repose. The granules showed satisfactory flow properties, compressibility and drug content. The granules were punched into tablet and tablet was evaluated for the parameter like thickness, weight variation, hardness, friability, and drug content. All six formulations showed acceptable pharmacotechnical properties and with required hardness, weight variation, friability and drug content. The hardness was slightly higher with tablet containing hydrophobic polymer (Ethyl cellulose).
The in-vitro drug release was studies with USP XXII dissolution apparatus in both simulated gastric fluid and intestine fluid for a period of 12hr. the results of dissolution studies indicated that formulation (Losartan potassium with SCMC with Ethyl cellulose) produced sustained effect with 74.19% of drug release over a period of 12hr. in comparison to other formulation. The mechanism of drug release was diffusion coupled with erosion.
It can be concluded that the polymer plays a major role in the design of sustained release matrix tablet. Combination of different classes of (Hydrophilic and Hydrophobic) polymer is essential to get acceptable pharmacokinetic profile in the fluctuating in vivo environment.
Bibliography
Refrencess
- US Pharmacopoeia NF United states pharmaceutical Convention, INC,Rockville. MD. p- 2498-2500. 2007
- Atul Kuksal, Ashok K. Tiwary, Narendra K. Jain and Subheet Jain Formulation and In Vitro, In Vivo Evaluation of Extended- release Matrix Tablet of Zidovudine: Influence of Combination of Hydrophilic and Hydrophobic Matrix Formers. AAPS Pharm sci tech; 7(1) 1, 2006.
- K. Ebube, A.B. Jones, Sustained release of acetaminophen from a heterogeneous mixture of two hydrophilic non-ionic cellulose ether polymer, Int. J. Pharm. 272, 19–27,2004.
- J. Lee, S.G. Ryu, J.H. Cui, Formulation and release characteristics of hydroxypropylmethyl-cellulose matrix tablet containing melatonin, Drug Dev. Ind. Pharm. 25, 493–501,1999.
- Sako, T. Sawada, H. Nakashima, S. Yokohama, T. Sonobe, Influence of water soluble fillers in hydroxypropylmethylcellulose matrices on in vitro and in vivo drug release, J. Control. Release 81,165–172, 2002.
- Reddy KR, Srinivas Mutalik, and Srinivas Reddy Preparation of once-daily sustained-release matrix tablets of nicorandil. AAPS Pharm sci tech; 4(4), 61. 2003.
- Pharmacopoeia of India. New Delhi: Ministry of Health and Family Welfare, Government of India, Controller of Publications; 1996.
- Chopra S, Gayathri V. Patil, Sanjay K. Motwani release modulating hydrophilic matrix systems of losartan potassium. Eu. J.of Pharmaceutics and biopharmaceutics 66, ,73-82, 2007.







