Manuscript accepted on :November 30, 2009
Published online on: 17-11-2015
Plagiarism Check: Yes
Pradeep Tyagi¹* and Pankaj Sharma²
¹Department of Pharmaceutics, Bhupal Noble’s College of Pharmacy, Udaipur. Rajasthan India.
²Raj Kumar Goel Institute of Technology, 5 Th Mile Stone, Delhi-meerut Road, Ghaziabad India.
Abstract
Gastro retentive systems can be retained in the stomach for a long time so Glipizide hypoglycemic agent used as in gastro retentive formulations in the form of pellets can be developed by using extrusion-spheronization method. Pellets were found to be satisfactory in terms of floatability and drug release behavior. Floatation was achieved during the entire study period of different formulations. The surface morphology of the optimized formulation shows the pellets were smooth and spherical shape. The best formulation was considered to be the optimized one. Improve floating time, sustained drug delivery was observed when the polymer HPMC, HPC, MCC used in the ratio of 28%, 11%, 11%, respectively.
Keywords
Glipizide; Extrusion-Spheronization; Pellets; Polymers HPMC; HPC; MCC
Download this article as:| Copy the following to cite this article: Tyagi P, Sharma P. Controlled Oral Drug Delivery of Hypoglycemic Agent Through Particulate System. Biomed Pharmacol J 2009;2(2) |
| Copy the following to cite this URL: Tyagi P, Sharma P. Controlled Oral Drug Delivery of Hypoglycemic Agent Through Particulate System. Biomed Pharmacol J 2009;2(2).Available from: http://biomedpharmajournal.org/?p=808 |
Introduction
The term controlled release implies a system that provides continuous delivery of the drug for a predetermined period with predictable and reproducible kinetics and a known mechanism of release. Also included in this term are systems that provide control over movement of dosage form through the GI tract and / or deliver the drug to a specific area within the GI tract for either local or systemic effect. Among all routes of drug administration that have been explored for the development of controlled-release (CR) systems, the oral route has by far achieved the most attention and success. This is due, in part, to the ease of administration as well as to the fact that gastrointestinal physiology offers more flexibility in dosage form design than most other routes.
Often one encounters additional factors, including the disease being treated, the patient, and length of therapy. Given that it is not practical to alter the physicochemical characteristics of the drug, design of controlled-delivery systems generally optimizes dosage form characteristics relative to the GI environment.
The pattern of motility is however distinct in the two states. During the fasting state an inter digestive series of electrical events take place, which cycle both through stomach and intestine every 2 to 3 hours. This is called the interdigestive myloelectric cycle or migrating myloelectric cycle (MMC).
Controlled drug delivery systems that can be retained in the stomach for a long time are known as gastro retentive systems. Such retentive systems are important for drugs that are degraded in intestines or for drugs like antacids or certain enzymes that should act locally in the stomach. If the drugs are poorly soluble in the intestine due to alkaline ph, gastric retention may increase solubility before they are emptied, resulting in improved bioavailability. Such systems are advantageous in improving gastrointestinal absorption of drugs with narrow absorption windows as well as for controlling release of the drugs having site specific absorption limitation. Such systems are useful in cases where the drug is best absorbed from the upper part of the intestine
Drug released from the devices that achieve gastric retention would be emptied along with the gastric contents over a long period and would thus, be present at the main absorption site, the small intestine, for a longer time. This prolonged gastric retention could improve bioavailability and reduce drug wastage. Therefore, a gastro retentive system will thus, be helpful in achieving maximum effect of the drug
Such system can not be used in case of drugs that induce gastric lesion or for drugs that are unstable in the acidic environment of stomach. Many times drug incorporation is a major problem with such delivery systems. It is not easy to design and fabricate such systems as retention of these systems depends upon factors such as gastric motility, ph and presence of food.
Aim and Ojectives
The aim of this study is to identify and optimize ratio of floatable excepients /drug along with and other formulation factors which could be easily and effectively controlled through extrusion and spheronization process to yield glipizide pellets with maximum floating time and prolonged release.
Methods Employed
Formulation of Pellets
Glipizide pellets were prepared by extrusion-spheronization method. The excipients and drug was mixed to homogeneity (10 min). Isopropyl alcohol/water (9:1) was used as the pelletizing agent and a wet mass of suitable consistency for extrusion was formed. The damped mass was extruded through a 1.0-mm mesh screen in an extruder at 15 rpm. [Caleva Ltd, UK]. The extrudates were spheronized for specified time (minutes) at specified rpm in a Caleva model 120 spheronizer. The obtained pellets were dried in a fluidized bed dryer for specified drying time and temperature.
Study of the floatation behavior of granules
The floatation studies were carried out to ascertain the floating behavior of various polymer combinations. Static volume Beaker method was initially used to have an idea of the floatation behavior of the proposed dosage form. A set of formulations were prepared by extrusion-spheronization technique in which the drug along with the polymers were taken. These were then individually placed in separate beakers having equal volume of hydrochloric acid buffer pH 1.2.
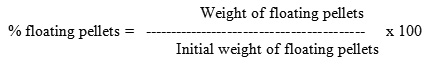
Bulk density, tapped density and Hausner factor
Granules (1 gm) were placed in a 10 ml volumetric cylinder and their volume was determined. The bulk density was calculated as g/cm3.
The cylinder was then tapped 500 times and the volume was determined again afterward to calculate the tapped density.
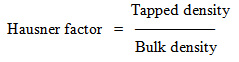
Particle size distribution
Particle size distribution was determined by sieve analysis. One gram of granules were put on the top of the sieve with a series of openings ranging from 1.41 mm (sieve no. 14), 1.00 mm (sieve no. 18), 0.84 mm (sieve no. 20), 0.41mm (sieve no 40) to 0.20mm (sieve no. 60). The results are reported as percentage of weight retained on each sieve size.
In vitro release rate study
In vitro dissolution studies were carried out in USP dissolution apparatus II using 900 ml of the dissolution medium [in case simulated gastric condition, hydrochloric acid buffer pH 1.2 was selected] at 37±0.5 oC temperature and at 50 rpm. The 5 ml sample was removed at each predetermined time interval from vessel and the fresh medium was replaced. The samples were filtered using millipore filter assembly and the drug content was estimated by HPLC from the standard area plot of glipizide. For further characterization of delivery system its behaviour was also evaluated in simulated intestinal conditions as the study was conducted in phosphate buffer( pH 6.8) following the same dissolution conditions.
Scanning electron microscopy
The surface morphology of the optimized pellets were seen by scanning electron microscopy (SEM). Scanning electron microscopy was done on a LEO 435 BP instrument. Pellets were fixed on aluminium stubs sputter coated with gold and examined under the microscope
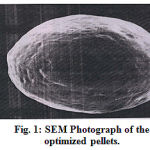 |
Figure 1:SEM Photograph of the optimized pellets.
|
Inference
The surface morphology of the optimized formulation determined by scanning electron microscopy revealed that the surface of pellets was almost smooth and shape was spherical.
The drug sample was characterized for physical appearance, odour, MP, loss on drying etc. in order to establish the authenticity of the drug.
The drug was identified on UV & FTIR basis. The drug exhibited absorption maxima at 237 nm in the range 200-300 nm which was same as mentioned in literature. On the basis of these studies, it was proved that the drug sample was authentic.
Procedure For Formulation Of Pellets
Excipients and drug was accurately weighed, screened by passing through sieve No. 40 and then admixed in a mortar for 10 minutes. Isopropyl alcohol and water in the ratio of (9:1) was added in sufficient quantity to make wet mass in the following formulation design sets. The wet mass which was subjected to extrusion ( at various speeds) to give extrudes which transformed to pellet upon spheronization( at various time and speed of spheronization), were dried at a temperature of 60 oC overnight. The various parameters of extrusion and spheronization were needed to optimize on the basis of yield, size and shape and floating tendency.
Procedure For Release Characteristic
Weigh accurately the amount of pellets (equivalent to 5.0 mg of glipizide) and placed in a USP type II dissolution vessel, containing 900 ml of dissolution medium, keeping 50 rpm (HCl buffer pH 1.2 or phosphate buffer pH 6.8) and maintaining the dissolution condition as given in USP monograph for glipizide tablet. The sample (5.0ml) was withdrawn from the predetermined intervals from the dissolution vessel. Fresh medium was replaced to keep the volume of medium constant. Sample containing glipizide from HCl buffer pH 1.2 obtained after dissolution were analyzed by HPLC method whereas sample from dissolution studies conducted in phosphate buffer pH 6.8 were analyzed by using the standard plot drawn spectrophotometrically at a wavelength of 275 nm. Dissolution data was reported in cumulative amount dissolved and mean percentage release.
Experimental Tables
Table 1 : Formulation of pellets of different ratio of drug / polymer
| Formulation of pellets of different ratio of drug / polymer | |||||
| Ingredients | Formulation code | ||||
| 7A | 7B | 7C | 7D | 7E | |
| DRUG | 58.8% | 58.8% | 60% | 60% | 60% |
| HPC-M | – | – | 10% | 11% | 11% |
| HPC-LH21 | 5.8% | 8.8% | – | – | – |
| HPMC | 23.5% | 17% | 15% | 22% | 28% |
| MCC | 11.76% | 21.2% | 15% | 7% | 11% |
| D/P RATIO | 1.5:1 | 1.5:1 | 1.5:1 | 1.5:1 | 1.5:1 |
| FLOATING TIME (min) | 60 | 60 | 240 | 420 | 420 |
| T 2hr | 40% | 80% | 100% | 60% | 50% |
Table 2:Pellets Characterization of formulations
| Pellets Characterization | |||||
| Formulation code | 7A | 7B | 7C | 7D | 7E |
| Bulk Density | 0.57 | 0.56 | 0.56 | 0.55 | 0.57 |
| Tapped Density | 0.66 | 0.64 | 0.65 | 0.64 | 0.63 |
| Compressibility index | 13.6 | 12.5 | 13.8 | 14 | 9.5 |
| Hausners ratio | 1.15 | 1.14 | 1.16 | 1.16 | 1.10 |
| Angle of repose | 19.2 | 19.3 | 19.1 | 19.2 | 19.1 |
| Friability % | 0.78 | 0.81 | 0.79 | 0.8 | 0.78 |
| Loss on drying % | 1.1 | 1 | 1.1 | 1 | 1 |
Table 3: In vitro drug release study.
| In –vitro drug release characteristic of various formulation in HCl buffer pH 1.2 | |||||
| Time (min) | % Cumulative drug release | ||||
| 7A | 7B | 7C | 7D | 7E | |
| 0 | 0 | 0 | 0 | 0 | 0 |
| 30 | 17 | 38 | 66 | 33 | 20 |
| 60 | 28 | 60 | 83 | 45 | 34 |
| 90 | 36 | 70 | 94 | 59 | 43 |
| 120 | 40 | 80 | 100 | 60 | 50 |
| 150 | 47 | 90 | – | 66 | 56 |
| 180 | 53 | 98 | – | 73 | 63 |
| 240 | 64 | – | – | 86 | 75 |
| 300 | 73 | – | – | 96 | 84 |
| 360 | 82 | – | – | 100 | 92 |
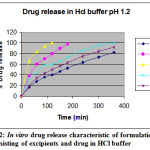 |
Figure 2: In vitro drug release characteristic of formulation consisting of excipients and drug in HCl buffer.
|
Table 4: Stability assessment of various parameters of formulation (7E)
|
S.No |
Property |
Formulation 7E |
|
| Before stability test
|
After stability assessment | ||
| 1 | Assay | 100.157 (±0.457) | 98.97 (±0.354) |
| 2 | Floating time | 420(±10) minutes | 450(±20) minutes |
| 3 | T50% | 125(±15) minutes | 130(±20) minutes |
Results and Discussions
Preformulation studies
Glipizide a potent new sulphonylurea indicated for the control of type II diabetic mellitus acts by partially blocking potassium K+ channels in the beta cells of the islets of Langerhans with signaling leading to an increase in intracellular calcium which itself initiate more insulin release from each beta cell. Glipizide was selected as the model drug for floatable delivery system since it is well absorbed after oral administration with half life of 2-4 hours. It also matched perfectly with this platform as it possesses less density.
The drug sample as received was characterized for its identity, purity and the bulk characterization. Bulk characterization studies of drug involved the measurement of bulk density, tapped density and flow property measurement.
Formulation studies
The floating behavior, drug release, characterization and micromeritic properties were reviewed for selection of excipient having floating capabilities. The design of floating device revealed that the formulation strategy should begin with appraisal of the density function of individual component as it serves a crucial factor which directly affects the floating behavior of the formulation. The density of drug and excipients (polymeric or non polymeric) were considered for the formulation of floating pellets as this would have direct influence on the floating behavior of the formulations. The excipients and drug were so selected to cause this system to float infinitely thus controlling the drug release up to the desired time with minimum variation in the drug concentration level in the blood/plasma.
Optimized formulation
The formulation 7E was prepared using only those fractions of the selected polymers which showed promising floating and release behavior. Only the optimized processing step, conditions were used in preparation of 7E. The optimized quantity of granulating fluid i.e. 9:1 (I.P.A: WATER) was selected for the formulation of 7E. The granulated mass was extruded and spheronised at an optimized step of 5, 10, 20 rpm and 500, 1000, 1500 rpm respectively. A yield of 80% was obtained at 5 rpm which however increased to 87% as the speed was raised to 10 rpm, but when the speed was further raised to 20 rpm the yield did not improve. The effect of spheronization on the pellets geometry was studied at two different times i.e. 2 min & 5 min , keeping the speed of spheronization constant i.e. 500, 1000, 1500 rpm. The best step was 500 rpm for 5 min, as a yield of 87 % was observed with minimum wastage of extrudes and also the pellets were spherical in shape at this speed and time combination. However at the same rpm (500) for 2 min partial spheronization was observed. These pellets had comparatively larger size 1000 µm with the 90 % yield but imperfection in shape, size of the pellets was observed .At 1000 rpm, the extrudes took less time to transform the extrudes into pellets with the product yield of only 75 % and spherical shape of average particle size was only 900µm. On further increase of spheronization time, pellets become compact agglomerated spheres (made up of 3 -4 pellets) with a size 2000 -2500µm. The higher rpm (1500) caused the agglomerates to generate powdered particles as it is a surface process where a tangential force is applied through out extrudes which transform its shape into the spherical pellet. At higher speed frequent scraping was required which would affect the desired spherical shape of the pellets. Hence a balance between spheronization time and spheronization speed would definitely affect the shape and size yield of pellets .So similar justification could be given in case of pellets prepared at 1500 rpm and kept at two different levels of spheronization time. Lower yield (50%) was obtained at 5 minutes whereas 70% yield was obtained at 2 minutes spheronization time. The similar explanation could be given for shape at less and high spheronization time.
The effect of drying time was also studied at two different levels, 6 hr dried pellets was more friable compared to 12 hr dried pellets.
The drug release characteristic of optimized formulation was studied in phosphate buffer ph 6.8 and much sustained effect was obtained. Applying the various releases modeling predicted that optimized pellets formulation followed release of zero order model in HCl buffer pH 1.2.
The ICH guidelines were followed to carry out the stability testing of drug product. The different evaluation parameters assessed were % weight change (effect of humidity), assay of drug, specific appraisal parameters for which it was designed (i.e. floating time) and the release characteristic in HCl buffer and phosphate buffer pH 6.8. The pellets were found to be stable.
Conclusion
The main interest in such a dosage form resulted from its ability to invariably maximize drug absorption by increasing the retention time of the drug in the stomach. Gastroretentive formulations in the form of pellets can be developed by using extrusion-spheronization method. Floatation was achieved during the entire study period of formulation 7E. The surface morphology of the optimized formulation 7 E showed that the pellets were smooth and spherical shape. Formulation 7E was considered to be the optimized one. Formulation 7E meets all the desired attributes of a floating drug delivery system.
References
- J. Moes, Floating delivery and other potential gastric retaining systems, current status on targeted drug delivery to the gastrointestinal tract, Capsugel Library, 1993, pp. 97-112.
- Baret, L., and Remon, J.P.1993. Influence of amount of granulation liquid on the drug release rate from pellets made by extrusion-spheronization. Int. J. Pharm. 95, 135-141.
- Chavanpatil, M., Jain, P., chaudhari, S., Shear, R., Vavia, P., 2005.Development of sustained release gastro retentive drug delivery system for ofloxacin In vitro and in vivo evaluation. Int. J. Pharm. 304, 178-184. Chavanpatil, M., Jain, P., chaudhari, S., Shear, R., Vavia, P., 2006. Novel sustained release, swellable and bioadhesive Gastroretentive drug delivery system for ofloxacin. Int. J. Pharm. 316, 86-92.
- Martin, Physical Pharmacy, IVth ed., Lea Febiger, Philadelphia, 1993, pp. 431-432.
- Agyilirah, G.A., Green, M., Ducret, R. and Banker, G.S., “Evaluation of the gastric retention properties of a cross linked polymer coated tablet versus those of non disintegrating tablet”, J. Pharm., (1991) 75: 2541-247.
- Durig, T., Fassihi, R., 1999. An investigation into the erosion behavior of a high drug – load (85%) particulate system designed for an extended -release matrix tablet .Analysis of erosion kinetics in conjunction with variation in lubrication , porosity, and compaction rate .J. Pharm .PHarmacol . 51, 1085-1092.
- Fielden, K.E., Newton, J.M., and Rowe,R.C., The effect of lactose particle size on the extrusion properties of microcrystalline cellulose –lactose mixtures .J. Pharm. Pharmacol., 41 (1989) 217-221.
- Gröning, R., Cloer, C., Georgarakis, M., and Rotraut S., Compressed collagen sponges as gastroretentive dosage forms: In vitro and in vivo studiesEuro. J. Pharm. Sci. 30, 1-6.
- Akabuga, J., Preparation and evaluation of controlled release of furosemide microspheres by spherical crystallization. Int. J. Pharm. 53 (1989) 99-105.
- S. Soppimath, A.R. Kulkarni, T.M. Aminbhavi, Development of hollow microspheres as floating controlled-release systems for cardiovascular drugs: preparation and release characteristics, Drug Dev. Ind. Pharm. 27 (2001) 507.
- Whitehead, J.T. Fell, J.H. Collett, H.L. Sharma, A.M. Smith, Floating dosage forms: an in vivo study demonstrating prolonged gastric retention, J. Control. Release 55 (1998) 3-12.
- Lachman, L.; Lieberman, H.A.; Schwartz, J.B. Pharmaceutical Dosage Form: Tablets, 2nd Ed.; Marcel Dekker, Inc.: New York, 1990; 107-348.
- Merck, 1976. The Merck Index. An Encyclopedia of chemicals and Drugs, 9th Merck & Co., Rahway, NJ.
- Ohta, T., Miyajima, K., Komuro, G., Furukawa, N., Yonemori, F., 2003. Antidiabetic effect of chronic administration of JTT-608, a new hypoglycemic agent, in diabetic Goto- Kakizaki rats. Euro. J. Pharmacol. 476, 159-166.
- Qui, Y., Gupta, P., Briskin, J., Cheskin, H., Semla, S., 1996. Sustained release multiparticulate formulations of Zileuton. I. In vitro and in vivo evaluation. Int. J. Pharm. 143, 179-185.
- Robinson, R.L., and Hollenbeck, R.G., Manufacture of spherical acetaminophen pellets: Comparison of rotary processing with multiple – step extrusion and spheronization. Pharm. Tech., 15 (1991) 48-56.
- Singh, B.N., Kim, K.H., 2000.Floating drug delivery system: an approach to oral controlled drug delivery via gastric retention .J. Controlled Release 63, 235-259.
- Sonaglio, D., Bataille, B., Ortigosa, C., Jacob, M., 1995. Factorial design in the feasibility of producing Microcel MC 101 pellets by extrusion \ spheronization. Int. J. Pharm. 115, 53-60.
- The Merck Index, XII, Merck & Co., Inc., Whitehouse Station, NJ, 1996.
- Timmermans, J., Moes, A.J., 1994. Factors controlling the buoyancy and gastric retention capabilities of floating matrix capsules: new data for reconsidering the controversy. J. Pharm. Sci. 83, 18-24.
- Wilson CG, Washington The stomach: its role in oral drug delivery. In: Rubinstein MH, ed. Physiological Pharmacetical:Biological Barriers to Drug Absorption. Chichester, UK: Ellis Horwood; 1989:47Y70.
(Visited 130 times, 1 visits today)







