S. A. Mastan, P. Janaki Ramayya, L. Mutyala Naidu and K. Mallikarjuna
Post Graduate Department of Biotechnology, D.N.R. College, P.G. Courses and Research Centre, Bhimavaram - 534 202 India.
Corresponding Author E-mail: shaikmastan2000@yahoo.com
Abstract
The antimicrobial activity of hexane, chloroform and methanol extracts from Mucuna pruriens leaves has been evaluated, invitro against Xanthomonas compestris, Agrobacterium rhizogenes, Pseudomona aeruginosa, Alternaria solani, colletotricum capsici, Rhizoctonia solani, Penicillium expansium, Fusarium oxysporum, Ustilago maydis and Curvalaria lunata. All extracts except Mucuna Pruriens leaves exhibited antimicrobial activity against different species of bacteria and fungi. But hexane extract does not showed any effect on bacteria tested the methanol extract of Mucuna Pruriens showed highest antimicrobial activity against all the bacterial and fungal species tested. Whereas chloroform extracts showed moderate antimicrobial activity against both bacterial and fungal species. The results of present study indicate that Mucuna Pruriens plant may be a good candidate as antimicrobial agent.
Keywords
Mucuna Pruriens; antimicrobial activity; Microorganisms
Download this article as:| Copy the following to cite this article: Mastan S. A, Ramayya P. J, Naidu L. M, Mallikarjuna K. Antimicrobial Activity of Various Extracts of Mucuna Pruriens Leaves. Biomed. Pharmacol. J.2009;2(1) |
| Copy the following to cite this URL: Mastan S. A, Ramayya P. J, Naidu L. M, Mallikarjuna K. Antimicrobial Activity of Various Extracts of Mucuna Pruriens Leaves. Biomed. Pharmacol. J.2009;2(1). Available from: http://biomedpharmajournal.org/?p=605 |
Introduction
Microbial infections pose a health problem throughout the world with the alarming increase in the rate of infection by antibiotic resistant microorganisms7. The increasing resistance of most synthetically derived antimicrobial agents in of utmost concern. The development of drug resistance in human pathogens against commonly used antibiotics has necessitated a search for new antimicrobial substances from other sources including plants13. The WHO has also recommended the evaluation of the effectiveness of plants in conditions where we lack safe modern drugs. Evaluation of antimicrobial medicinal plants in essential because phototherapy is cheaper and locally available.
To whom all correspondence should be addressed
Mucuna pruriens (Linn.) belongs to Fabaceae, commonly known as common cowith, cowhage, kavach, velvet bean, kapikachhu and naikaranam. It is an indigenous leguminous plant, is well known for producing itch. It is one of the most popular drugs in the Ayurvedic system of medicine. All parts of Mucuna pruriens posses valuable medicinal properties3. The roots are bitter, sweet thermogenic, emollient, stimulant, purgative and diuretic. The seeds are astringent, laxative, antihelmentic, alexipharmic and tonic22. The leaves are broadly ovate, elliptic or rhomboid ovate, unequal at base. Leaves used as Aphrodisiac, antihelmentic, tonic and are useful in Stomach ulcers, Inflammation, Helminthiasis, Cephalalgia and general debility21. Mucuna pruriens posses a wide range of pharmacological activities such as anti inflammatory12 neuroprotective activity16 anti oxidant activity23 anti diabetic7,8 anti protozoal activity19 antimicrobial activity10. Therefore, in the present investigation efforts have been made to study the antimicrobial activity of hexane, chloroform and methanolic extracts from Mucuna pruriens leaves against various bacterial and fungal species in vitro.
Materials and Methods
Collection of plant material
Fresh leaves of Mucuna pruriens were collected from Bhimavaram, West Godavari District, Andhra Pradesh. The leaves were washed thoroughly with running tap water, shade dried and grinded to powder using a table model grinder. Sieving method was used to separate the fine plant powder and stored in air tight bottle.
Preparation of extracts
Plant extracts were prepared accordingly to the method of Alade and Irobi2 with little modification.10gms shade dried, powdered plant material were soaked separately in 100ml of hexane, chloroform and methanol for 72 hours with periodical stirring and mixing. Then the extracts were separately filtered through cheese cloth. The crude extracts were evaporated to dryness under reduced pressure at 400C. The residues were weighed and appropriate quantities were dissolved in Dimethyl sulfoxide (DMSO) to obtain a final concentration of 1mg/ml.
Microorganisms used
The following test organisms namely Xanthomonas compestris, Agrobacterium rhizogenes, Pseudomona aeruginosa, Alternaria solani, colletotricum capsici, Rhizoctonia solani, Penicillium expansium, Fusarium oxysporum, Ustilago maydis and Curvalaria lunata were used in this study. These bacterial and fungal strains were obtained from Institute of Microbial Technology (IMTECH), Chandigarh, India. The obtained bacterial species were grown in nutrient broth (Himedia Pvt. Ltd, Bombay) at 370C and maintained on nutrient agar slants at 40C and stored at -200C. The fungal cultures were maintain in sabouraud dextrose agar and stored at -200C.
Antibacterial activity
Antibacterial activity was carried out by Agar cup diffusion method15. The nutrient agar was dissolved in distilled water and PH of the medium was adjusted to 7.0. The medium was cooled to 40-500C. 20 ml aliquots of inoculated nutrient agar poured into sterile Petri plates and allowed to solidify. In each plate 3 wells with 6 mm in diameter were made using a sterile cork borer. 45-50ml (100mg/ml) of different extracts was filled in each well by using Finn pipette adjustable volume digital pipette. After that the plates were incubated at 370C for 24 hrs. Three replicates were maintained for each extract against each of the test organism. Simultaneously control was also maintained without extract. After 24 hours of incubation the inhibition zones were measured by OMNICON antibiotic zone reader and average mean values were presented in table.
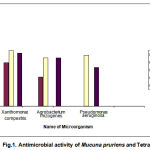 |
Figure 1
|
Antifungal activity
The antifungal activity was studied employing the standard cup-plate method16. Instead of nutrient agar Sabouraud dextrose agar was used. The inoculated plates were incubated at 250C for 3 to 4 days. Two drops of 5 % streptomycin sulphate was added to the agar for fungal medium to prevent bacterial growth. After 4 days of incubation, the inhibition zones were measured and results are presented in the table. DMSO served as negative control while tetracycline and Nystatin as positive control for bacteria and fungi, respectively.
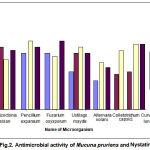 |
Figure 2
|
Results and Discussion
The antimicrobial activity of Mucuna Pruriens leaves against tested microorganisms is summarized in table 1&2. All extracts except hexane of Mucuna Pruriens leaves shows antibacterial activity against tested bacteria. Hexane extract does not have any effect on tested bacteria. But it has moderate antifungal activity against fungal species tested. The methanolic extract of Mucuna Pruriens showed highest antimicrobial activity against all the bacterial species tested. Chloroform extract showed moderate antimicrobial activity against tested microorganisms. Antimicrobial activity of Mucuna Pruriens in our study may be due to the secondary metabolites. It is evident from the literature that the Mucuna Pruriens possess a wide range of pharmacological uses such as anti-inflammatory, neuroprotective, antioxidant activity, antidiabetic and antiprotozoan activity.
Table 1: Antimicrobial activity of various extracts of Mucuna pruriens leaves.
| Microorganisms | ||||
| Zone of inhibition in mm± SD | ||||
| S.No | Name of extract | Xanthomonas | Agrobacterium | Pseudomonas |
| compestris
|
rhizogenes
|
aeruginosa | ||
|
1. |
Hexane extract |
— |
— |
— |
| (50ml /well) | ||||
| 2. | Chloroform extract | 18±0.63 | 12±0.54 | 20±0.36 |
| (50ml /well) | ||||
| Methanol extract | ||||
| 3. | (50ml /well) | 23±0.29 | 20±0.36 | 21±0.54 |
| Tetracycline | ||||
| 4. | (+ve control, 30mg/ml) | 22±0.28
|
20±0.54
|
16±0.54 |
Values represent the mean ± SD of 3 replicates.
Table 2: Antimicrobial activity of various extracts of Mucuna pruriens leaves.
| Microorganisms | ||||||||
| Zone of inhibition in mm± SD | ||||||||
| S.No | Name of | Rhizoctonia | Pencillium | Fusarium | Ustilago | Curvalaria | Alternaria | Colletotrichum |
| extract | solani
|
expansum
|
oxysporum
|
maydis
|
lunata
|
solani
|
capsici
|
|
|
1. |
Hexane extract |
— |
18±0.49 |
18±0.36 |
12±0.28 |
— |
12±0.36 |
— |
|
|
(50ml /well) |
|
|
|
||||
| 2.
|
Chloroform extract | 20±0.45
|
—
|
—
|
18±0.27
|
12±0.27
|
—
|
15±0.36
|
| (50ml /well) | ||||||||
| 3. | Methanol extract | 16±0.36
|
22±0.36
|
17±0.36
|
20±0.49
|
21±0.49
|
20±0.36
|
25±0.28
|
|
|
(50ml /well) | |||||||
| 4. | Nystatin | 19±0.63 | 20±0.36 | 22±0.28 | 18±0.54 | 21±0.27 | 18±0.28 | 27±0.49 |
| (+ve control, 30mg/ml) | ||||||||
* Diameter of well = 6 mm
Values represent the mean ± SD of 3 replicates.
Rajeswar et al20 reported that the tissues of Mucuna Pruriens showed antibacterial activity against gram positive and gram negative bacteria. The wound healing potency of crude extract of leaf, stem, seed, bark, kernel and leaves of Mucuna momosperma was also reported by Manjunath et al15. In the present study, it has also been observed that, the chloroform extract of Mucuna Pruriens exhibited pronounced activity against microorganisms tested and it may be due to the presence of terpenoids, steroids, flavonoids, tannis and kerotonins. This study gets support from the work of Ahmad and Beg1who reported that the flavonoids are found to be effective antimicrobial substances against and wide range of microorganisms, probably due to their activity to complex with extra cellular and soluble protein and to complex with bacterial cell wall24.
Conclusion
All these findings in the present study raise some interesting expectation about the antimicrobial activity of the Mucuna Pruriens and it is possible that the identification and elucidation of the constituents in this plant may lead to the development the new and effective drugs for treating various diseases.
References
- Ahmed I and Beg AG Antimicrobial and Phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. J Ethnopharmacol 2001 74: 113-123.
- Alade Pi and Irobi ON Antimicrobial activities of crude leaf extracts of /acarypha wilkewsiana. Ethano Pharmacol. 1993 39:171-174.
- Caius JF The Medicinal and poisonous legumes of India. Scientific Publishers, Jodhpur. 1989 pp. 70-71.
- Chhabra SC, Shao JF and Mshiu EN Antifungal activity among traditionally used herbs in Tanzania. The Dar Medicinal Journal 1982 9:68-73.
- Cole MD The significance of the terpenoides in Labiatae. In: Harley, R.M., Reynolds, T. (Eds.), Advances in Labiatae Science. Royal Botanic Gardens, Kew, 1992 pp. 315-324.
- Cowan M Plant products as antimicrobial agents. Clin. Microbiol Rev 1999 12(4):564-582.
- Davies J. In activation of antibiotics and the dissemination of resistance genes. Science. 1994 264:375-382.
- Dawan BN, Dubey MO and Gesa AA Screening of Indian plants for biological activity. Part 9. Ind J Expt Biol 1980 18: 594-606.
- Dixon R, Dey P and Lamb C Phytoalexins: Enzymology and molecular biology. Adv Enzymol 1983 55:1-69.
- Ekanem Ap, Objekezie A, Klos W and Knojof K Effects of crude extracts of pruriens (Fabaceae) and Carica papaya (Caricaceae) against the protozoan fish parasite Icchthyophthiris multifiliis. Parasitol Res 2004 92 (5): 361-366.
- Eloff JN which extract should be used for the Screening and isolation of antimicrobial components from plants? J Ethnopharmacol 1986 60: 1-8.
- Hishikar R, Shastry S, Shinde S and Guptha SS Preliminary photochemical and anti-inflammatory activity of seeds of M. pruriens. Ind J Pharmacol 1989 13 (1):97-98.
- Balaraju, S. Arokiyaraj, P. Agastian, N. Thomas Pulraj and S. Ignacimuttu Antibacterial activity of swertia chissata buch. Hams. A highly valuable Medical Herb. J. Pure & Applied Microbiol., 2008 Vol 2(1): 223-226.
- le Grand A, Wondergem PA, Verpoorte R and Pousset JL Anti-infectious phytotherapies of the treesavannah of Senegal (West-Africa) II. Antimicrobial activity of 33 species. J Ethnopharmacol 1988 22(1):25-31.
- Manjunatha BK, Patil HSR, Vidya SM, Kekuda TRP, Mukunda S and Divakar R Studies on the antibacterial activity of Mucuna monosperma Indian Drugs 2006 43: 150- 152.
- Manyam BV, Dhanasejkaran M and Hare TA Neuroprotective effects of the antiparkinson drug Mucuna pruriens. Phytother Res 2004 18 (9): 706-712.
- Perez C, Pauli M and Bazerque P An Antibiotic assay by the well agar method. Acta Biologiac Medicine experimentalis 1990 15: 113-115.
- Perilla M J Manual for the laboratory identification and antimicrobial susceptibility testing of bacterial pathogens of public health importance in developing world. WHO 2003 pp. 209-214.
- Rathi SS, Grover JK and Vats V The effects of Momardica charantia and pruriens in experimental diabetes and their effect on key metabolic enzymes involved in carbohydrate metabolism. Phytother Res 2002 16(8): 774-777.
- Rajeshwar Y, Guptha M and Mazumder UK In vitro lipid peroxidation and antimicrobial activity of M. pruriens seeds. Iranian J Pharmacol Ther 2005 4 (1): 32-35.
- Sathyanarayanan L and Arulmozhi S Mucuna pruriens – A Comprehensive Review. Pharmacognosy Reviews Vol 1, Issue 1. Jan-May. 2007 157-162.
- Taylor L The Healing power of Rainforest Herbs, 2005 p.444.
- Tripathi YB and Upadhyay AK Antioxident property of Mucuna pruriens Linn. Curr Sci 2001 80 (11): 1378.
- Tsuchiya H, Sato M, Miyazaki T, Fujiwara S, Tanigaki S, Ohyama M, Tanaka T and Iinuma M Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. J Ethnopharmacol 1996 50(1):27-34.
- Williams LAD, Vasques E, Reid W, Porter R, and Kraus W Biological activities of an extract from Cleome viscose L. (Capparaceae). Naturwissenschaften 2003 90:468-472.







