Manuscript accepted on :Septembe 26, 2008
Published online on: 09-12-2015
Plagiarism Check: Yes
Lakhdar Sekhri*, Mohammed Lazhar Kadri, Choula Samira and Moussa Senigra
Department of Chemistry, Faculty of Sciences, University of Kasdi Merbah, Ouargla
Corresponding Author E-mail:sekhril@yahoo.fr
Abstract
The novel penicillin derivatives Amp-1 and Amx-1 have been synthesised via diazotising aniline with sodium nitrite in a medium of hydrochloric acid and the resulting phenyldiazonium chloride couples to ampicillin and amoxicillin. Esterification of ampicillin and amoxicillin with ethanol and isopropanol yielded the penicillin derivatives Amp-2, Amp-3, Amx-2, and Amx-3. The penicillin derivative Oxa-1 was successfully synthesized by nitration of oxacillin. These penicillin derivatives (except Oxa-1) showed significant activity against all the bacteria tested, Escherichia Coli, Streptococcus pneumonia, Protius, Staphylococcus aureus and Pseudomonas.
Keywords
Penicilin derivatives Amp-1; Amx-1; Antibacterial activity
Download this article as:| Copy the following to cite this article: Sekhri L, Kadri M. L, Samira C, Senigra M. Synthesis of penicillin derivatives and study of their biological antibacterial activities . Biomed. Pharmacol. J.2008;1(2) |
| Copy the following to cite this URL: Sekhri L, Kadri M. L, Samira C, Senigra M. Synthesis of penicillin derivatives and study of their biological antibacterial activities . Biomed. Pharmacol. J.2008;1(2). Available from: http://biomedpharmajournal.org/?p=2429 |
Introduction
Since the 1940’s, chemists have developed all sorts of highly effective antibiotics (Sulfa drugs, Pinicillins, Tetracyclines, and others that are effective) against bacterial infections and viral infections-from Influenza and the common cold to the more serious herbs infections and AIDS (Acquired Immune Defections Syndrome). The viral infections account for about 60% of illnesses, contrasted with about 15% for bacterial infections. Since any antiviral agent must selectively kill pathogens in the presence of other living cells, the progress with these drugs has been slower and more difficult.
In recent years there has been a flood of papers describing the synthesis of new antibacterial compounds and isolation of some natural products and study of their biological antimicrobial activities (triazolo-thiadiazinyl comarins,1 p-bromo and p-nitrophenylselenocyanate,2 pyrazoline-5-ones,3 thiacarbamides,4 substituted benzimidazole derivatives,5 and others.6-8 The penicillin is one of the most widely used antibiotics and its antibacterial activity against both gram-positive and gram-negative organisms.9,10 The penicillin has two heterocyclic rings, the smaller, β-lactam, of which is crucial to biological activity and contain bulky side chains attached to the 6-aminopenicillanic acid.11-13 The establishment of the precise structure of penicillin was very difficult and was completed only in 1945, when Dorthy Hodgkin (Nobel Prize of chemistry 1964) brought the final tests thanks to work of diffraction of x-rays (Scheme-1) .
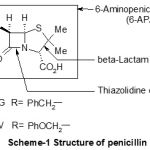 |
Scheme 1:
|
The molecular skeleton lets foresee that it probably derives from two amino acids, in fact cysteine and valine what was indeed proven. Its general structure is shown in Scheme-2.
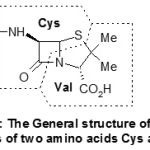 |
Scheme 2:
|
Now we found that the introduction of diazo (-N=N-) and carboxylate groups in the penicillin brought a high degree of antibacterial activity for this drug.
Results and Discussion
The strategy we have adopted for the synthesis of these novel penicillin derivatives consists of the following steps: (i) introducing the bulky group to a penicillin; this can be achieved by diazotising aniline with sodium nitrite in a medium of hydrochloric acid and the resulting phenyldiazonium chloride couples to ampicillin in order to obtain Amp-1. The novel penicillin derivative, Amx-1, was obtained in similar way to that of Amp-1 but the amoxicillin was used instead of ampicillin. The purpose of introducing the phenyldiazo group, Ph-N=N-, to ampicillin and amoxicillin as bulky groups is to prevent the anchoring of penicillin in the enzymatic pocket. Therefore the selected strategy to solve this problem consisted in preventing penicillin from reaching the active site of the penicillinase. A way of making a success of this objective would be to place a bulky group on the side chain of penicillin. The aforementioned bulky group can then play the role of a shield to protect itself from the penicillinase (Scheme-3).
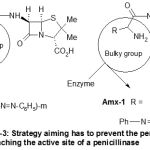 |
Scheme 3:
|
In addition, the functional group –N=N- may have some benefit as long as the antibacterial activity is concerned. In the last decade, a variety of reactive dyes14 and diazo reactive dyes15,16 have been established as a major group for fixation to cellulose.
The β-lactamases are enzymes which are produced by the bacteria’s penicillino-resistantes and catalyse the reaction presented in Scheme-4, i.e. the same opening of β-lactame cycle, in short the same deactivation of the penicillin as that which caused the acid hydrolysis.
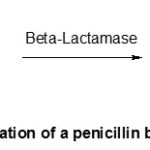 |
Scheme 4:
|
Ampicillin and amoxicillin can cause diarrhoea, because of their poor absorption on the level of the intestinal wall, which causes a disturbance of the intestinal flora. This problem of poor absorption on the level of the intestinal wall comes from nature dipole of these molecules since they contain at the same time a free amino group and a carboxylic acid function. This problem can be solved by calling upon one prodrug which one of these entities polar is masked by a protective group. This group will be then removed by biotransformation as soon as the prodrug is reabsorbed at the intestinal level as shown in Scheme-5.
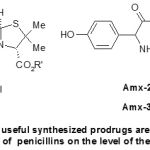 |
Scheme 5:
|
The penicillin derivative, Oxa-1, was obtained by nitration of oxacillin (Scheme-6). Unfortunately the functional group NO2 has no benefit as long as the antibacterial activity is concerned.
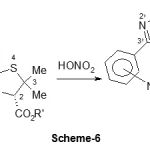 |
Scheme 6:
|
All 1H NMR spectra of ampecillin derivatives Amp-1, Amp-2 and Amp-3, and amoxicillin derivatives Amx-1, Amx-2 and Amx-3 possess two characteristic sharp singlet peaks at 1.35-1.89 ppm that are belong to the two methyl protons of ampecillin and amoxicillin. The other peak around 4.50 ppm corresponds to the C-H proton next to the acid group. The spectra reveal a peak at 6.9-7.5, which is due to the protons of aromatic group. These results agree well with other works reported recently by Mojtaba Shamsipur,17 considering the fact that a constant shifts is observed in all peaks due to the use of CDCl3 instead of D2O. Mojtaba Shamsipur and co-workers used deuterium oxide/formic acid (56/44) throughout as a proper solvent and found that the 1H NMR spectrum of ampecillin possess two characteristic sharp singlet peaks at 0.640 and 0.675 ppm that are belong to the two methyl protons. The other peak around 3.64 ppm corresponds to the C-H proton next to the acid group. The spectra reveal a peak at 6.76 for Ar-H. These results agree well with other works12,55 and in all their spectra, they observed two sharp singlet peaks at 4.78 and 7.63 ppm corresponding to H2O and formic acid, HCOOH, respectively. The biological medium is usually in an aqueous form, and for this reason, D2O was selected for all of the 1H NMR analyses performed in the previous work.1 In addition, the penicillins have limited stability, especially in organic solvents, and produce different degradation products18,19 and for this reason, all 1H NMR spectra of ampecillin derivatives Amp-1, Amp-2 and Amp-3, and amoxicillin derivatives Amx-1, Amx-2 and Amx-3 do not indicate any signals for the proton next to the acid group and the protons of β–lactam.
Experimental
Synthesis
General
All 200 MHz 1H NMR spectra were run on a Bruker AC 200 Spectrometer. the NMR spectra were recorded using CHCl3 as internal standard. Infra red spectra were recorded using a Perkin-Elmer 783 spectrometer equipped with a PE 600 data station. Melting points were determined using an Electrothermal melting point apparatus and were uncorrected. Column chromatography was conducted on precoated aluminium sheets (60 F 254) with a 0.2mm thickness (Aldrich Chemical Co.).
Preparation of 6-(amino-p-azophenylbenzylformamido)-3, 3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid (Amp-1)
Aniline (0.88 g, 9.46 mmol) was dissolved in warm mixture of 2.7 ml of concentrated hydrochloric acid and 2.7 ml of water contained in a 100 ml beaker. The beaker was placed in an ice-salt bath, and cooled to 0 – 5°C. A cooled solution of sodium nitrite (0.67 g , 9.71 m mol) in 3.4 ml of water was added slowly. It is essential that the temperature be kept between 3 and 5°C during the diazotization, other wise tarry by products are formed.
Meanwhile powdered ampicillin (4 g, 9.92 mmol) was dissolved in 8 ml of 10% sodium hydroxide contained in a 250 ml beaker and the temperature was then lowered to 0°C by means of an ice-salt bath. The diazonium salt solution was then added over a 15 minutes period and the temperature was kept bellow 0°C, so that any temperature rise caused by the coupling reaction would not be too drastic, and also to ensure complete reaction. Addition of ampicillin caused an immediate deep-red colour to be formed. Stirring was continued for 1h at room temperature, and the solution was added to water (200 ml). The red precipitate was filtered off and washed by adding 100 ml of NaOH (5%) and dried in an oven at 80°C; this preparation was repeated several times to improve the yield. The red solid (2 g, 3.94 mmol, 50%), m. p. 125-135°C was identified by spectroscopic data:
1H NMR (CDCl3): δ 1.52 (6H, s, 2 CH3), 1.94 (1H, s, CH), 2.09-2.11 (3H, d, 3 CH), 2.7 (2H, s, NH2), 3.1-3.5 (1H, br. s, NH), 7.40-7.70 (6H, m, Ar-H), 7.80-7.90 (2H, m, Ar-H). IR (KBr, cm-1): 3500-2500 (br., OH), 2860-2980 (CH aliphatic), 1770 (C=O, acid), 1680 (C=O, amide), 1605 (N=N).
Preparation of ethyl 6-(amino-p-azophenylbenzylformamido)-3, 3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate (Amp-2)
A dry 250 ml, two necked-round bottomed flask equipped with a rubber septum, a magnetic stirrer and reflux condenser was charged with (4g, 9.94 mmol) of ampecillin in (125 ml) of absolute ethanol and (2ml) of concentrated sulphuric acid. The reaction mixture was refluxed on a water bath with gentle stirring for 4hrs. The solvent was removed under reduced pressure on a rotavapor to give 4g of yellow crude material. To this crude material was added concentrated solution of sodium carbonate (25 ml) and allowed to stand to room temperature overnight. Water (50 ml) was added and the yellow precipitate was filtered off; washed thoroughly 5 times with 50 ml portions water. The pure yellow needles were dried in an air oven at 80 °C. These yellow needles, m. p. 170-175°C of Amp-2 (2.5g, 56%) has the spectroscopic properties:
1H NMR (CDCl3): δ 1.40-1.50 (9H, t, CH3), 4.50 (4H, q, CH2, 2CH), 7.50 (5H, m, Ar-H), 8.2 (1H, s, NH). IR (KBr, cm-1): 3050 (Ar-H), 2850-2970 (CH aliphatic), 1726(C=O, ester), 1270 (C-O).
Preparation of isopropyl 6-(amino-p-azophenylbenzylformamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate (Amp-3)
Ampecillin (1.7g, 4.2 mmol) was esterified using isopropanol (110 ml, 144.1 ml) and concentrated sulphuric acid (2ml) to Amp-3 in similar way to Amp-2. Amp-3 was obtained as orange needles (0.7g, 36.04%), m.p. 180-182 °C. The product has the spectroscopic properties:
1H NMR (CDCl3): δ 1.40-1.50 (9H, t, CH3), 4.50 (4H, q, CH2, 2CH), 7.50 (5H, m, Ar-H), 8.2 (1H, s, NH). IR (KBr, cm-1): 3060 (Ar-H), 2850-2970 (CH aliphatic), 1735(C=O, ester), 1650 (C=O, amide), 1275 (C-O).
Preparation of (2S, 5R, 6R) 6-(amino-3-azophenyl-4-hydroxybenzylformamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid (Amx-1)
Aniline (6.35 g, 68.28 mmol) was dissolved in warm mixture of 20 ml of concentrated hydrochloric acid and 20 ml of water contained in a 800 ml beaker. The beaker was placed in an ice-salt bath, and cooled to 0 – 5°C. A cooled solution of sodium nitrite (5.15 g, 90.35 mmol) in 30 ml of water was added slowly. It is essential that the temperature be kept between 3 and 5°C during the diazotization, otherwise tarry by products are formed.
Meanwhile powdered amoxicillin (10.5 g, 25.06 m mol) was dissolved in 15 ml of 10% sodium hydroxide contained in a 600 ml beaker and the temperature was then lowered to 0°C by means of an ice-salt bath. The diazonium salt solution was then added over a 15 minutes period and the temperature was kept bellow 0°C, so that any temperature rise caused by the coupling reaction would not be too drastic, and also to ensure complete reaction.
Addition of amoxicillin caused an immediate deep-red colour to be formed. Stirring was continued for 1h at room temperature, and the solution was added to water (200 ml). The red precipitate was filtered off and washed by adding 100 ml of NaOH (5%) and dried in an oven at 80°C . The red solid (3.57 g, 6.82 mmol, 34%), m. p. 141-143°C was identified by spectroscopic data:
1H NMR (CDCl3): δ 1.50 (2H, s, NH2), 1.89 (6H, s, 2CH3), 6.93-7.50 (8H, m, Ar-H), 7.80-7.90 (2H, m, Ar-H), 8.00 (1H, d, NH, amide). IR (KBr, cm-1): 3000-3300 (br.s, OH), 2900 (CH aliphatic), 1732 (C=O, acid), 1680 (C=O, lactame), 1590 (N=N), 1275 (C-O), 11140 (C-N).
Preparation of (2S, 5R, 6R) ethyl 6-(amino-3-azophenyl-4-hydroxybenzylformamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate (Amx-2)
Amoxicillin (5.1g, 12.17 mmol) was esterified using ethanol (50 ml) and concentrated sulphuric acid (2ml) to 3b in similar way to that of a2. 3b was obtained as orange needles (1.89, 37.2%), m.p. 200-2005 °C. The product has the spectroscopic properties:
1H NMR (CDCl3): δ 0.9 (3H, t, CH3), 1.2 (2H, q, CH2), 1.8 (2H, s, NH2) 1.35 (6H, s, 2CH3), 7.3 (4H, m, Ar-H). IR (KBr, cm-1): 3000-3300 (br.s, OH), 2900 (CH aliphatic), 1732 (C=O, ester), 1680 (C=O, β-lactam), 11605 (C=C, Ar), 11140 (C-N), 1020 (C-O).
Preparation of (2S, 5R, 6R) isopropyl 6-(amino-3-azophenyl-4-hydroxybenzylformami do)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate (Amx-3)
Amoxicillin (5.5g, 13.12 mmol) was esterified using isopropanol (50 ml) and concentrated sulphuric acid (2ml) to 4b in similar way to that of a2. 4b was obtained as yellow needles (2.09g, 38.16%), m.p. 160-1170 °C. The product has the spectroscopic properties:
IR (KBr, cm-1): 3380 (br.s, OH), 3080 (CH, Ar), 2900 (CH aliphatic), 1780 (C=O, β-lactam), 1720 (C=O, ester), 1680 (C=O, 1230 (C-O).
Preparation of (2R, 5R, 6S)-3, 3-dimethyl-6-[5’-methl-3’-o-and/or p-nitrophenyl-1, 2-oxazole-4-carbonyl)amino]-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid (Oxa-1)
(4.0 g, 9.13 mmol) of oxacillin was dissolved in 15 ml of distilled water in a 250 ml necked-round bottomed flask equipped with a rubber septum, a magnetic stirrer and reflux condenser.
Meanwhile 1ml of concentrated nitric acid and (1ml) of concentrated sulphuric acid contained in a 250 ml beaker and then 10 ml of distilled water was added; the temperature was lowered to 25-30°. The resulting nitro solution was then added over a 15 minutes period and the reaction mixture was refluxed on a water bath with gentle stirring for 1hr and the temperature was kept bellow 60°C so that any temperature rise would cause dinitration and trinitration, and above 25°C, to ensure complete reaction. The pale yellow precipitate was filtered off and washed by adding 100 ml of NaOH (5%) and then washed again by diluted solution of HCl (10-4mol/l); dried in an oven at 80°C. The pale yellow solid of Oxa-1 (2.83g, 70.75%), was identified by comparison its IR and 1H NMR spectra with that of an authentic sample; the I.R. spectrum revealed the presence of nitro group: IR (KBr, cm-1) 1550 and 1350.
Microbiological studies
Antibacterial activity of all the newly synthesized compounds Amp-1, Amp-2 and Amp-3 was studied using the disc diffusion method. The bacterial strain used was Escherichia Coli, Staphylococcus Aureus and Streptococcus pneumonia.
The compounds were tested at a concentration of 400 µg/ml and were prepared in diluted solution of NaOH (0.7mmol/l). The Petri dishes used for antibacterial screening were incubated at 37 1°C for 24hrs. The results were compared to ampicillin by measuring zone of inhibition in mm using well plate method. Three determinations antibacterial activity of Amp-1, Amp-2 and Amp-3 are summarized in table-1 and Fig-1
Table 1 : Antibacterial activity concentration – 400 µg/ml.
| S Compounds Diameter of zone of inhibition in (mm)
No E.Coli S.Aureus Streptococus pnemonia |
| 1 Amp1 20 – –
2 Amp2 – – 17 3 Amp3 38 – 20 4 Ampecillin – 40 38 |
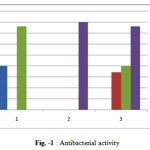 |
Figure 1 : Antibacterial activity 1 Escherichia Coli 2 Staphylococcus Aureus 3 Streptococcus pneumonia.
|
The newly synthesized compounds Amx-1, Amx-2 and Amx-3 were also tested at a concentration of 8000 µg/ml and were prepared in diluted solution of NaOH (0.7mmol/l). The results were compared to amoxicillin by measuring zone of inhibition in mm using well plate method. Three determinations antibacterial activity of Amx-1, Amx-2 and Amx-2 are summarized in table-2 and Fig-2
Table 2: Antibacterial activity concentration – 8000 µg/ml.
| S Compounds Diameter of zone of inhibition in (mm)
No E.Coli S.Aureus Protieus Pseudomonas |
| 1 Amx1 30 20 20 –
2 Amx2 32 23 12 – 3 Amx3 18 18 15 – 4 Oxa-1 – – – – 5 oxacillin – – – – 6 Amoxicillin – – – – |
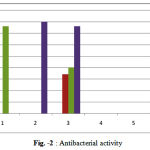 |
Figure 2 : Antibacterial activity1 Escherichia Coli 2 Staphylococcus Aureus 3 Protieus 4 Pseudomonas.
|
In microbiological study the penicillin derivatives showed significant activity against all the bacteria tested. These drugs showing significant bacterial activity are due to the presence of protective groups.
Acknowledgements
The authors are thankful to Dr. Ali Lounes and the staff of analytical and spectroscopic services of the Chemistry Department, University of Rennes, France and the staff of analytical and spectroscopic services of the Chemistry Department, University of Manchester, UK for their assistance.
References
- S. Jayashree, A.R. Sahu, M. Srinivasa Murthy and K.N. Venugopala, Asian J. Chem., 19 (1), 73 (2007).
- Cete, H. Ozkan, F. Arslan, Y.Yildirir and A. Yasar, Asian J. Chem., 19 (1), 550 (2007).
- Shiradkar, R. Kale, B. Baviskar and R. Dighe, Asian J. Chem., 19 (1), 449 (2007).
- T.Tayade, J. S. Waghmare and S. U. Patile, Asian J. Chem., 19 (1), 443 (2007).
- Algul, N. Duran and G. Gulbol, Asian J. Chem., 19 (4), 3085 (2007).
- Singh, J. Gautam, A. K. Jain and P. K.Mishra, Oriental Journal of Chemistry, 23 (2), 641 (2007).
- Suresh Kumar, R. Rajesh and Annes A. Siddiqui, Oriental Journal of Chemistry, 22(3), 641 (2006).
- Byczynska, I. Gajewski, A Bromiskirski, B. Carlson, R. Kozba, W. Majewska, I. Kups and J. Szkiela, Pol PL 173, 542 (1998).
- Byczynska, I. Gajewski, A Bromiskirski, B. Carlson, R. Kozba, W. Majewska, I. Kups and J. Szkiela, Chem. Abstr., 129, 204 (1998).
- M. A. Nissen, J. Chromatogr. A 812, 53 (1998).
- F. Wishart, J. Am. Vet Med Assoc., 185, 1106 (1984).
- L. Tyczkowska, R. D. Voyksner, A. L. Aronson, J. Chromatogr. 594, 195 (1991).
- M. Siegel, R. K. Isensee, D. J. Beck, Anal. Chem. 59, 989 (1987).
- S. Frank, S. C. Patrick, J. Chromatogr, A 812, 99 (1998).
- R. Mehta, R.R. Shah, S.K. patel and K. C. Patel, Acta Cienc. Indica, 30C, 9 (2004).
- S. Patel, P.S. Patel, K.C. Patel and S. K. Patel, Asian J Chem., 14, 420 (2002).
- Shamsipur, Z. Talebpour, H. R. Bijanzadeh, S. Tabatabaei, Journal of Pharmaceutical and Biomedical Analysis, 30, 1075 (2002).
- L. Tyczkowska, R. D. Voyksner, R. Strauk, A. L. Aronson, J. Chromatogr. 594, 195 (1992).
- Marzo, N. Monti, M. Ripamonti, J. Chromatogr. 507, 235 (1990).







