Manuscript accepted on : January 06, 2009
Published online on: --
Plagiarism Check: Yes
Milind Pande1* and Anupam Pathak2
1NRI Institute of Pharmaceutical Sciences, 3 Sajjansingh Nagar, Opp. Patel Nagar, Raisen Road, Bhopal (India)
2Department of Pharmacy, Barkatullah University, Hoshangabad Road, Bhopal (India).
Corresponding Author E-mail:milindpandey2006@rediffmail.com
Abstract
The leaves of Chinopodium album Linn. Family Chinopodiaceae have been used in Ayurvedic medicine since ancient times for the treatment of male sexual disorders. The present study is aimed to investigate the effect of ethanolic extract of bathua on general mating behaviour, libido, potency along with its likely gastric ulceration and adverse effects on sexually normal male albino mice. The suspension of the extract was administered orally at the dose of 100, 250, and 500 mg / kg, to different groups of male mice (n = 6) once a day for seven days. The female albino mice involved in mating were made receptive by hormonal treatment. The general mating behaviour, libido and potency were determined and compared with the standard reference drug sildenafil citrate. The probable gastric ulceration and adverse effects of the extract were also evaluated. Oral administration of the extract significantly increased the Mounting Frequency, Intromission Frequency; Intromission Latency, Erections as well as aggregate of penile reflexes and caused significant reduction in the Mounting Latency and Post Ejaculatory Interval. The most appreciable effect of the extract was observed at the dose of 500 mg/kg. The test drug was also found to be devoid of any conspicuous gastric ulceration and adverse effects. The results indicated that the ethanolic extract of Chinopodium album Linn. Family Chinopodiaceae produced a significant and sustained increase in the sexual activity of normal male mice, without any conspicuous gastric ulceration and adverse effects. Thus, the resultant aphrodisiac effectivity of the extract lends support to the claims for its traditional usage in sexual disorders.
Keywords
Chenopodium album; aphrodisiacs; sexual behavior; sex stimulant; mounting frequency
Download this article as:| Copy the following to cite this article: Pande M, Pathak A. Sexual Function Improving Effect of Chenopodium Album (Bathua sag) in Normal Male Mice. Biomed. Pharmacol. J.2008;1(2) |
| Copy the following to cite this URL: Pande M, Pathak A. Sexual Function Improving Effect of Chenopodium Album (Bathua sag) in Normal Male Mice. Biomed. Pharmacol. J.2008;1(2). Available from: http://biomedpharmajournal.org/?p=441 |
Introduction
A genus of strong smelling herb commonly known as pigweed in English and distributed through out world. About 21 species occur in India of which few have been introduced. Some are cultivated for vegetable, and few for grains. In Hindi common name is Bathua sag. A polymorphous, olive green to green, erect herb, up to 0.5 ft in height, and found wield in altitude of 700 meters and cultivated through out India. Stems rarely slender, angled, often striped green, red or purple; leaves rhomboid, deltoid to lanceolate, upper entire, lower toothed or irregularly lobed, extremely variable in cultivated forms, 10-15 cm long, petioles often as long as thick blade; flowers in clusters forming a compact or loosely panicle spikes in axils; utricles with round, compressed, shining black seeds, possessing sharp margins.1
The herb is a common weed during summer and winter in waste places and in the field of wheat, barley, mustard, gram and reduces their yield. The weeds are low growing while the cultivated plants are tall and leafy. The young plant of not more than 20 cm is much esteemed as a potherb. The tender shoots are eaten raw in salad or with curd; they are also cooked as vegetable or the cooked shoots are mixed with curd and eaten. The dried herb is stored for future use. It is also used as fodder; pigeons consume the plant in large quantities. The leaves are rich in potassium and vitamin C.
The leaves of Chinopodium album known as bathua sag is being used in traditional medicines. It has been found to have antipruritic and antinociceptic 2, sperm immobilizing agent 3, cryptomeridiol and 8- alpha-acetoxycyryptomeridiol as growth promoting activity. 4 It has been found to have flavonoid as phenolic amide 5, hypotensive in activity 6, saponin 7, rich in iron content 8, cinnamic acid amide 9, alkaloid chinoalbicin 10, apocortinoid 11, xyloside 12, phenols and lignans. 13
Experimental
Plant material and extraction
The leaves Chinopodium album Linn. Family Chinopodiaceae collected in and around Bhopal were identified in Department of Pharmacy, Barkatullah University, Bhopal. A voucher specimen (No BUPH/4041C) was deposited in the department. Before going to animal studies permission of Internal animal ethics committee of Department of Pharmacy, Barkatullah University has been obtained by meeting on date 26/12/2006 Ref No BUPH/ IAEC / 7865. The Pharmacy Department of Barkatullah University is also approved for animal experimentation by (Letter No .444/01/C/CPSCEA date July 2001).
Preliminary Phytochemical studies
The powder of dried leaves was subjected to continuous Soxhlet extraction with various organic solvents such as petroleum ether (60-800 c), chloroform, benzene, methanol & ethanol respectively. After concentration and drying of each extract in vacuum desicator, identification of phytoconstituents was carried out using thin layer chromatography method by different detecting reagents.14
Chemicals used
Sildenafil citrate was purchased from Zydus Cadila, (Ahmadabad, India). Other drugs used were ethinyl oestradiol (Infar Limited, Calcutta, India), progesterone (Sun Pharmaceutical Industries Limited, Mumbai, India) and 5% xylocaine ointment (Astra IDL Limited, Bangalore, India)
Animals
Twelve weeks old male and female albino mice of wistar strain weighing 25–30 g were used for the study. They were housed singly in separate standard cages and maintained under standard laboratory conditions (temperature 24–28°C, relative humidity 60–70%, 12 hr light-dark cycle) with free access to solid pellet diet (Gold Mohar, Lipton-India) and water ad libitum throughout the study except during the experiment. The ethical committee of the Department for animal cares and use approved the study design.
Drug preparation
Since bathua leaves in ayurvedic medicine is orally administered, therefore, the ethanolic extract of bathua was suspended in distilled water using Tween 80 (1%) for oral administration as protocol given in (Table No 1). Sildenafil citrate and ethinyl oestradiol were also suspended in distilled water using Tween 80 (1%) separately, for oral use. Progesterone was dissolved in olive oil for subcutaneous injection. All the drug solutions were prepared just before administration.
Table 1: Protocol For Evaluation Of Sexual Behaviour Activity Of Leaves of CHENOPODIUM ALBUM.
| S NO. | DRUG CODE | DOSE | ANIMAL AVERAGE WEIGHT | DOSE ADMINISTERED |
| 1 | CA/BTH | 3 mg in 2 ml | 28 gm-32 gm | OD for 7 days |
| 2 | CA/BTH | 7.5 mg in 2 ml | 28 gm-32 gm | OD for 7 days |
| 3 | CA/BTH | 10 mg in 2 ml | 28 gm-32 gm | OD for 7 days |
| 4 | Alcohol | 6% in feeding bottle of water | 28 gm-32 gm | OD for 7 days |
| 5 | Standard | Sildenafil citrate orally at the dose of 5 mg/kg | 28 gm-32 gm | OD for 7 days |
| 6 | Control | Normal diet and only vehicle | 28 gm-32 gm | OD for 7 days |
Mating behaviour test
The test was carried out by the methods of Dewsbury and Davis Jr 15, Szechtman et al 16, modified by Amin et al. 17 Healthy and sexually experienced male albino mice (25–30g) that were showing brisk sexual activity were selected for the study. They were divided into 6 groups of 6 animals each and kept singly in separate cages during the experiment. Group 1 represented the control group, which received 10 ml/kg of distilled water orally. Groups 2–4 received suspension of the extract of clove orally at the doses of 100, 250 and 500 mg/kg, respectively, daily for 7 days at 15:00 h. Group 5 served as standard and given suspension of sildenafil citrate orally at the dose of 5 mg/kg, 1 h prior to the commencement of the experiment. Group 6 was fade with alcohol 6% in drinking feed bottle with normal diet. Since the male animals should not be tested in unfamiliar circumstances the animals were brought to the laboratory and exposed to dim light (in 1 w fluorescent tube in a laboratory of 14′ × 14′) at the stipulated time of testing daily for 6 days before the experiment. The female animals were artificially brought into oestrus (heat) by the Ratna Soorya et al 18 method (as the female mice allow mating only during the estrus phase) They were administered suspension of ethinyl oestradiol orally at the dose of 100 μg/animal 48 hr prior to the pairing plus progesterone injected subcutaneously, at the dose of 1 mg/animal 6 hr before the experiment. The receptivity of the female animals was confirmed before the test by exposing them to male animals, other than the control, test and standard animals. The most receptive females were selected for the study. The experiment was carried out on the 7th day after commencement of the treatment of the male animals. The experiment was conducted at 15:00 h in the same laboratory and under the light of same intensity. The receptive female animals were introduced into the cages of male animals with 1 female to 1 male. The observation for mating behaviour was immediately commenced and continued for first 2 mating series. The test was terminated if the male failed to evince sexual interest. If the female did not show receptivity another replaced her artificially warmed female. The occurrence of events and phases of mating were recorded as number of mounts before ejaculation or Mounting Frequency (MF), number of intromission before ejaculation or Intromission Frequency (IF), time from the introduction of female into the cage of the male up to the first mount or Mounting Latency (ML), time from the introduction of the female up to the first intromission by the male or Intromission Latency (IL), time from the first intromission of a series up to the ejaculation or Ejaculatory Latency (EL), and time from the first culation up to the next intromission by the male or Post Ejaculatory Interval (PEI). In the second mating series only the EL was recorded. The values for the observed parameters of the control, test and standard animals were statistically analyzed by using one-way analysis of variance (ANOVA) method.
Test for libido
The test was carried out by the method of Davidson 19, modified by Amin et al. Sexually experienced male albino mice were divided into 6 groups of 6 animals each and kept singly in separate cages during the experiment. Group 1 represented the control group, which received 10 ml/kg of distilled water orally. Groups 2–4 received suspension of the extract orally at the doses of 100, 250 and 500 mg/kg, respectively, once a day in the afternoon (15:00 h) for 7 days. Group 5 served as standard and given suspension of sildenafil citrate orally at the dose of 5 mg/kg, 1 h prior to the commencement of the experiment. Group 6 was fade with alcohol 6% in drinking feed bottle with normal diet. The female mice were made receptive by hormonal treatment and all the animals were accustomed to the testing condition as previously mentioned in mating behaviour test. The animals were observed for the Mounting Frequency (MF) on the evening of 7th day at 15:00 hr. retracting the sheath exposed the penis and 5% xylocaine ointment was applied 30, 15 and 5 min before starting observations. Each animal was placed individually in a cage and the receptive female mice were placed in the same cage. The number of mountings was noted. The animals were also observed for intromission and ejaculation. The MF in control, test and standard animals was statistically analyzed by employing one-way analysis of variance (ANOVA) method.
Test for potency
The effect of the test drug was studied according to the methods described by Hart and Haugen 20 and Hart 21, modified by Amin et al. The male animals were divided into 6 groups of 6 animals each and kept singly in separate cages during the experiment. Group 1 represented the control group, which received 10 ml/kg of distilled water orally. Groups 2–4 received suspension of the test drug orally at the doses of 100, 250 and 500 mg/kg, respectively, daily for 7 days. Group 5 received a suspension of sildenafil citrate orally at the dose of 5 mg/kg, 1 h before the commencement of the experiment. Group 6 was fade with alcohol 6% in drinking feed bottle with normal diet. On the 8th day, placing the animal on its back in a glass cylinder partial restraint carried out the test for penile reflexes. The perpetual sheath was pushed behind the glans by means of thumb and index finger and held in this manner for a period of 15 min. Such stimulation elicits a cluster of genital reflexes. The components was recorded Erections (E). The frequency of these parameters observed in control, test and standard groups was statistically analyzed by using one-way analysis of variance (ANOVA) method.
Test for ulcerogenecity
The male animals (25–30g) were divided into 5 groups of 6 animals each. Group 1 represented the control group, which received 10 ml/kg of distilled water. Groups 2–4 received suspension of the extract orally at the doses of 100, 250 and 500 mg/kg, respectively, daily for 7 days. Group 5 was fade with alcohol 6% in drinking feed bottle with normal diet. After the treatment, on 8th day all animals were killed and the stomach was then incised along the grater curvature and washed carefully with physiological saline. Any gastric lesions were observed immediately using a magnifying glass. The number of erosions per stomach was assessed for severity, according to score of Cioli et al 22 as (0) absence of lesion, vasodilation or up to 3 pinpoint ulcers, (1) for more than 3 pinpoint ulcers, (2) from 1 to 5 small ulcers (< 2 mm); (3) more than 5 small ulcers (< 2 mm); (4) 1 or more giant ulcers.
All treated mice were observed at least once daily for any overt sign of toxicity (salivation, rhinorrhoea, lachrymation, ptosis, writhing, convulsions and tremors), stress (erection of fur and exophalmia) and changes in behaviour (such as spontaneous movements in cage, climbing, cleaning of face). In addition food and water intake were noted.
Results and Discussion
Successive solvent extraction values in various organic solvent were observed as petroleum ether 3.53%, benzene 2.33%, chloroform 2.83%, acetone 2.66%, methanol 5.44% and ethanol 4.55% as shown in (Table no 2)
Table 2: Successive solvent Extraction of leaves of Chenopodium album Linn.
| S. No. | Solvents used | Colour & Consistency | Average extractive values in % w/w on dry weight basis |
| 1 | Petroleum Ether 40-60 | Black green oily mass | 3.53 |
| 2 | Benzene | Black Green sticky mass | 2.33 |
| 3 | Chloroform | Light green residue | 2.83 |
| 4 | Acetone | Yellow | 2.66 |
| 5 | Methanol | Yellow blackish mass | 5.44 |
| 6 | Ethanol | Brown dry mass | 4.55 |
The preliminary phytochemical studies with help of Thin Layer Chromatography method revealed that presence of alkaloid in chloroform, acetone, and methanol extract prominently. The flavonoid was present in chloroform, acetone, and ethanol respectively. The essential oil was observed in petroleum ether and benzene extract clearly. (Table No 3)
Table 3: Thin layer chromatography scheme used to detect various extracts of leaves of Chenopodium album Linn.
| Solvent system used | Detection Reagent | Observation | Inference | P | B | C | A | M | E |
| Ethyl acetate: Methanol: Water (75.5:13.5:10) | KOH | Red. (Vis)
Yellow |
Anthraquinone
Anthrone |
– | – | – | – | – | – |
| Vanillin sulphuric acid | Red/ yellow/brown/blue-green | Bitter principle | – | – | – | – | – | – | |
| Dragendorffs reagent | Orange Red (vis) | Alkaloid | – | – | + | + | + | – | |
| NP/PEG and UV | Yellow/green/orange | Flavonoid | – | – | + | + | – | + | |
| VS reagent | Blue (vis) | Saponin | – | – | – | – | – | ||
| Toluene : ethyl acetate (93: 7) | VS reagent | Red/ yellow/brown/blue-green | Essential oil | + | + | – | – | – | – |
| Hcl/Acetic acid | Blue brown | Valepotriate | – | – | – | – | _ | – | |
| NH3 / KOH | Light Blue brown | Coumarin | – | – | – | – | – | – |
The test drugs ethanolic extract of leaves of Chenopodium album clearly indicated that significant increase in Mounting frequency as in the group treated with test drug 250 mg dose 34.80±0.837 and in the group treated with 500 mg 48.4± 1.14 as compared to control 20.60± 1. 14. However, this activity was found to a higher extent in the group treated with the standard drug 48.80± 1.30.
The test drugs ethanolic extract of leaves of Chenopodium album clearly indicated that significant increase in Intromission frequency as in the group treated with test drug 250 mg dose 48.12± 1.99 and in the group treated with 500 mg 64.15± 2.65 as compared to control 19.33 ± 1.78. However, this activity was found to a higher extent in the group treated with the standard drug 64.01 ± 2.55.
The test drugs ethanolic extract of leaves of Chenopodium album clearly indicated that significant increase in Mounting latency as in the group treated with test drug 250 mg dose 81± 6.89 and in the group treated with 500 mg 92±2.55 as compared to control 70± 6.24. However, this activity was found to a higher extent in the group treated with the standard drug 90±6.15.
The test drugs ethanolic extract of leaves of Chenopodium album clearly indicated that significant increase in Intromission latency as in the group treated with test drug 250 mg dose 210.16 ±5.46 and in the group treated with 500 mg 238.00 ±5.86 as compared to control 125.5 ±4.53. However, this activity was found to a lower extent in the group treated with the standard drug 240.33 ±4.85.
The test drugs ethanolic extract of leaves of Chenopodium album clearly indicated that significant increase in Ejaculatory latency as in the group treated with test drug 250 mg dose 78.83 ± 0.89 and in the group treated with 500 mg 92.32 ± 0.10 as compared to control 51.63 ± 0.47. However, this activity was found to a lower extent in the group treated with the standard drug 93.85± 3.25.
The test drugs ethanolic extract of leaves of Chenopodium album clearly indicated that significant increase in Post ejaculatory index as in the group treated with test drug 250 mg dose 36 ± 1.31 and in the group treated with 500 mg 64±1.39 as compared to control 50 ± 13. However, this activity was found to a lower extent in the group treated with the standard drug 66 ± 1.26.
The test drugs ethanolic extract of leaves of Chenopodium album clearly indicated that significant increase in Erections as in the group treated with test drug 250 mg dose 9.57 ±0.20 and in the group treated with 500 mg 12.02 ±0.08 as compared to control 4.19 ±0.10. However, this activity was found to a lower extent in the group treated with the standard drug 12.05 ±0.069. (Table No 4)
Table 4: Effects of Ethanolic Extract of Chenopodium album (SEXUAL BEHAVIOURS).
| GROUPS | MF
(In No) |
IF
(In No) |
ML
(In Second) |
IL
(In No) |
EL | PEI | E (In No) |
| I) CONTROL | 20.60±
1. 14 |
19.33 ± 1.78 | 70±6.24 | 125.5 ±4.53 | 51.63 ± 0.47 | 50 ± 13 | 4.19 ±0.10 |
| II) STANDARD | 48.80+ 1.30 | 64.01 ± 2.55 | 90±6.15 | 240.33 ±4.85 | 93.85±3.25 | 66 ± 12 | 12.05 ±0.069 |
| III) 100 mg DOSE | 22.4±
1.14 |
43.67 ± 2.39 | 70±3.25 | 140.5 ± 5.24 | 59.75 ± 0.94 | 21+12 | 7.76 ±0.19 |
| Iv) 250 mg DOSE | 34.80±
0.837 |
48.12+ 1.99 | 81±6.89 | 210.169 ±5.46 | 78.83 ± 0.89 | 36 ± 13 | 9.57 ±0.20 |
| V) 500 mg DOSE | 48.4±
1.14* |
64.15+ 2.65* | 92± 2.55* | 238.00 ±15.86* | 92.32 ± 0.10* | 64+ 13* | 12.02 ±0.08* |
| VI) Alcohol | 11.60±
5. 14 |
9.23 ±2.78 | 35±3.12 | 65.5 ±2.53 | 25.63 ± 2.47 | 25 ± 3.45 | 2.45 ±0.10 |
Values are mean ±SEM of six animals
Statistical significance: *= p<0.01 comparison was done with their respective control group
Orientation Activity
Furthermore, our studies on orientational activities showed that the treated males increased anogenital sniffing, licking and mounting of females and enhanced genital grooming of their own. These animals also showed lake of interest in the external environment and their movements were to be restricted. (Table No 5).
Table 5: Effect of on orientational activities towards female, towards environment & towards self on 7-day administration in normal mice and treated mice.
|
Group (Dose\kg b. wt)
|
Towards female
(60 minutes) |
Towards environment
(60 minutes) |
Towards self
(60 minutes) |
||
| No. of licking | No. of anogenital sniffing | No. of climbing | No. of genital grooming | No. of non-genital grooming | |
| Control | 3.16 ±0.30 | 3.83 ±0.47 | 16.33 ±0.95 | 7.66 ±0.55 | 14.33 ±0.73 |
| Standard | 5.6 ±0.16 | 6.50 ±0.22 | 23.33 ±0.33 | 12.66 ±0.47 | 22.16 ±0.55 |
| II) 100 mg DOSE (CA/BTH) | 2.00 ±0.25 | 2.83 ±0.87 | 3.16 ±0.30 | 2.50 ±0.22 | 6.16 ±1.10 |
| II) 250 mg DOSE (CA/BTH) | 3.00 ±0.25 | 3.00 ±0.25 | 4.66 ±1.01 | 4.66 ±0.49 | 8.33 ±0.60 |
| II) 500 mg DOSE (CA/BTH) | 6.83 ±0.60* | 9.50 ±0.56* | 26.16 ±1.53* | 20.16 ±2.08* | 27.00 ±0.68* |
| III) Alcohol | 2.00 ±0.25 | 2.83 ±0.87 | 3.16 ±0.30 | 2.50 ±0.22 | 6.16 ±1.10 |
The ethanolic extract of leaves of Chenopodium album modified mice copulatory as well as orientational activities, the two main determinants measuring male sexual behaviour. The result of present study revealed a significant increase in penile errection and frequencies of mounting and intromission. The ejaculatory latencies were also prolonged in treated animals indicated improved sexual performance. The drug extract also enhanced orientational activities of males towards female mice showing vigorous anogenital investigatory behavior and enhanced self-orientation as evidenced by increased grooming of the genitals. Our present reports show the animals treated with Chenopodium album at two dose levels showed increasing in copulatory sexual behaviour and orientational activities in test drug treated mice. Hence, increased sexual performance in mice treated with Chenopodium album may be mediated through dopaminergic pathway. The important functions of dopaminergic pathways divide broadly into motor control (nigrostriatal system) behavioral effects (mesolimbic and mesocortical system), endocrine control (tuberohypophyseal system). Stimulation of effects of dopaminergic pathway causes a cessation of normal behavior (exploration and grooming) and the appearance of repeated “stereotyped” behavior (rearing, gnawing and so on). Hence our present reports evidenced that, increased orientational activity in animals treated with our plant may be mediated through dopaminigeric pathway. These finding support the use of this plant as sex stimulant. Our present findings suggest that plant Chenopodium album had an effective in treatment of sexual dysfunction in castrated mice and to improve sexual performance in normal mice.
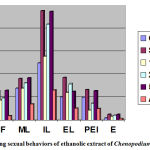 |
Graph 1: Graph showing sexual behaviors of ethanolic extract of Chenopodium album.
|
Summary and Conclusion
The present results indicated that the ethanolic extract of leaves of Chinopodium album Linn. Family Chinopodiaceae possesses potent sexual behaviour improving activity in normal male albino mice without any gastric ulceration and adverse effects and provided scientific evidence in favour of the claims made in ayurvedic medicine that the Chinopodium album Linn. Family Chinopodiaceae is clinically useful as sexual invigorator in males.
References
- Kirtikar K. R. and Basu B. D. Indian Medicinal Plants”, Vol III, 2 nd , Lalit Mohan Basu, Allahabad, 1964-1965.(1935).
- Dai Y.; Ye W. C.; Wang Z. T., Matsuda H.; Kubo, M.; But, P.P.H. Antipruritic and antinociceptive effects of Chenopodium album in mice. Journal of Ethnopharmacology, 81(2): 245-250 (2002).
- Kumar S, Biswas S, Mandal D, Roy HN, Chakraborty S, Kabir SN, Banerjee S, Mondal NB. Chenopodium album seed extract: a potent sperm-immobilizing agent both in vitro and in vivo. Contraception. 75(1): 71-8 (2007).
- Bera B., Mukherjee K.K., Ganguly S.N. Chemical investigation of the seeds of diploid cytotypes of Chenopodium album. Fitoterapia. 62(2): 178 (1991).
- Horio T., Yoshida K., Kikuchi H., Kawabata J., Mizutani J. A phenolic amide from roots of Chenopodium album. Phytochemistry. 33(4): 807-808 (1993).
- Gohar A. A., Elmazar M.M.A. Isolation of hypotensive flavonoids from Chenopodium species growing in Egypt. Phytotherapy Research. 11(8): 564-567( 1997).
- Lavaud, C., Voutquenne L., Bal P., Pouny I. Saponins from Chenopodium album. Fitoterapia. 71 (3): 338-340 (2000).
- Yadav S. K., Sehgal S. In vitro and in vivo availability of iron from Bathua (Chenopodium album) and spinach (Spinacia oleracea) leaves. Journal of Food Science and Technology. 39(1): 42-46 (2002).
- Cutillo F., Abrosca, B., Dellagreca M., Marino C.D., Golino A., Previtera, L., Zarrellia A. Cinnamic acid amides from Chenopodium album effects on seeds germination and plant growth. Phytochemistry. 64 (8): 381-1387 (2003).
- Cutillo F, D’Abrosca B, DellaGreca M, Zarrelli A. Chenoalbicin, a novel cinnamic acid amide alkaloid from Chenopodium album. Chem Biodivers. 1(10): 1579-83 (2004).
- DellaGreca M, Di Marino C, Zarrelli A, D’Abrosca B. Isolation and phytotoxicity of apocarotenoids from Chenopodium album. J Nat Prod. 67(9): 1492-5 (2004).12)
- Dellagreca M, Previtera L, Zarrelli A. A new xyloside from Chenopodium album. Nat Prod Res. 19(1): 87-90 (2006).
- Cutillo F, DellaGreca M, Gionti M, Previtera L, Zarrelli A. Phenols and lignans from Chenopodium album. Phytochem Anal. 17(5): 344-9 (2006).
- Wagner H, Bladt S, Zgainski FM. Plant Drug Analysis. Versa Berlin Publisher, 194, 291-304 (1989).
- Dewsbury D.A., Davis H.N. Jr. Effect of Reserpine on the copulatory behaviour of male rats. Physiol Behav. 5:1331–1333 (1970).
- Szechtman H, Moshe H, Rabi S. Sexual behavior pain sensitivity and stimulates endogenous opioid in male rats. Eur J Pharmacol. 70:279–285 (1981).
- Amin K.M.Y., Khan, M.N., Rahman S.Z., Khan, N.A.. Sexual function improving effect of Mucuna pruriens in sexually normal male rats. Fitoterapia. 67:53–58 (1996).
- Ratna Sooriya W.D., Dharmasiri M.G. Effect of Terminalia catappa seeds on sexual behaviour and fertility of male rats. Asian J Androl. 2:213–219 (2000).
- Davidson J.M. Sexology, sexual biology, behaviour and therapy: selected papers of Fifth World Congress of sexology Jerusalem 1981, Zewi H., Editor. Amsterdam, Holland. Excerpta Medica, Princeton – Oxford, 42–47 (1982).
- Hart B.L., Haugen C.M. Activation of sexual reflexes in male rats by spinal implementation of testosterone. Physiol Behav. 3:735–738 (1968).
- Hart B.L. Activation of sexual reflexes in male rats by dihydro testosterone but not estrogen. Physiol Behav. 23:107–109 (1979).
- Cioli V., Silvestrini B., Dordoni F. Evaluation of potential of gastric ulceration after administration of certain drugs, Exp Mol Pathol. 6:68–75 (1967).







