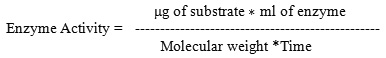Manuscript accepted on :October 15, 2008
Published online on: --
Plagiarism Check: Yes
V. Praveen Kumar¹*, K. Shrinivasulu² and P. Kalpana³
¹Lecturer, Department of Biotechnology, K.L College of Engineering, Green Fields, Vaddeswaram, Guntur District (India).
²Lecturer, Department of Biotechnology, K.L College of Engineering, Green Fields, Vaddeswaram, Guntur District (India).
³Department of Biotechnology, K.L College of Engineering, Green Fields, Vaddeswaram, Guntur District (India). Corresponding Author: vemuripraveen_bt@klce.ac.in
Abstract
Production of alkaline protease employing the laboratory isolate, Bacillus sp. under solid state fermentation (SSF) was optimized. The effect of wheat bran was examined. The appropriate Incubation time, Temperature, pH, Moisture content, Inoculums size, Surfactant and mutagen effect were determined. Maximum yields of enzyme were achieved by employing wheat bran as substrates in 0.1 M carbonate/bicarbonate buffer at pH 10 with 1:1.5 moisture content at 48 hrs. Inoculums size and buffer solution level were found to be 30% for wheat bran. The increased growth of bacterium of mutagenic activity is seen with acrylamide solution.
Keywords
Alkaline protease; SSF; NCIM
Download this article as:| Copy the following to cite this article: Kumar V. P, Shrinivasulu K, Kalpana P. Partial Purification and Optimization of Alkaline Protease from Bacterial ncim 2724 Species by Solid State Fermentation. Biomed. Pharmacol. J.2008;1(2) |
| Copy the following to cite this URL: Kumar V. P, Shrinivasulu K, Kalpana P. Partial Purification and Optimization of Alkaline Protease from Bacterial ncim 2724 Species by Solid State Fermentation. Biomed. Pharmacol. J.2008;1(2). Available from: http://biomedpharmajournal.org/?p=465 |
Introduction
Enzymes are proteins that catalyze (i.e. accelerate) chemical reactions. In these reactions, the molecules at the beginning of the process are called substrates, and the enzyme converts them into different molecules, the products. Almost all processes in the cell need enzymes in order to occur at significant rates. Since enzymes are extremely selective for their substrates and speed up only a few reactions from among many possibilities, the set of enzymes made in a cell determines which metabolic pathways occur in that cell.
The field of use for industrial enzymes has now extended to almost all industries handling organic compounds. Enzymes are used variably, for example, as ingredients of detergents, reagents for analysis of drugs or blood components, food use or food additives, fiber processing use or pulp processing in the paper industry, and environmental purification use.
Solid state Fermentation involves the cultivation of microorganisms on moist solid substrates in the absence of free flowing water. Solid state fermentation (SSF) holds tremendous potential for the production of enzymes. It can be of special interest in those processes where the crude fermented product may be used directly as the enzyme source. The selection of a substrate for enzyme production in a SSF process depends upon several factors, mainly related with cost and availability of the substrate, and thus may involve screening of several agro-industrial residues.
Materials and Methods
Bacterial Process
Organism
The organism used in the present study is Bacillus subtilis NCIM (National Collection of Industrial Microorganisms) No. 2724, purchased from NCL – Pune. The culture was routinely maintained in Nutrient Agar medium [Peptone (10gm/lt), Beef Extract powder (10gm/lt), NaCl (5gm/lt), Agar (20gm/lt)]. Fresh slants are prepared every fortnight to maintain the culture. The inoculation was done by adding 2% of culture broth grown for 24 hrs from slants.
Fermentation conditions
Commercial wheat bran (10gms) and Rice Bran (10gms) were finely mixed with 15ml of distilled water in a 250ml Erlenmeyer flask and sterilized at 15lb/in2 for 20min. After cooling, the flasks were inoculated with 10% inoculum size. The contents were mixed thoroughly, incubated at 37°C for 24 hrs and assayed for enzyme activity.
Optimization studies
Growth periods ranging from 24 – 72 hrs, growth temperature varying from 30 – 70°C,
effect of varying pH, effect of varying moisture content, effect of varying inoculum size, effect of varying substrate concentration, effect of surfactants, effect of different carbon and nitrogen sources, effect of CaCO3 on enzyme activity were checked. Also the activity of crude enzyme, enzyme precipitated by ammonium sulphate and acetone were checked for its activity. The mutated species of the same strain were studied using mutagens like Ethidium Bromide, incubation in UV light, Acrylamide.
Enrichment of Rice and wheat brans with different carbohydrate sources was established at the concentration of 0.04gm/1gm of substrate. The water insoluble carbohydrates were thoroughly mixed with the substrates before moistening, and the water soluble ones are dissolved in distilled water and used for moistening of substrates. The enrichment with different nitrogen sources was done at the concentration of 0.02gm/gm of substrate similar to carbohydrates.
Extraction of Enzyme
Bacterial extraction
After 24 hrs of incubation, the brans were mixed with 15ml distilled water (1:1.5 moisture content) and homogenized by centrifugation at 10,000 rpm for 15min. This yields the supernatant which is used as a crude enzyme source. It can further be purified by partial purification methods like precipitation by Ammonium Sulphate and Acetone.
Analytical Methods
Partial Purification
Ammonium Sulphate Precipitation
To 1ml of crude enzyme extract add 1ml of Tris-HCl Buffer and 2ml of 50% Ammonium Sulphate and centrifuge at 5000 rpm for 10min.
To the pellet add 1ml of Tris-HCl Buffer and 2ml of 60% Ammonium Sulphate and centrifuge at 5000rpm for 10min.
To get the highest purity of enzyme the concentration of Ammonium Sulphate is varied from 50% to 80% and repeated.
The final pellet is resuspended in 1ml Tris–HCl Buffer and used as enzyme source.
Acetone Precipitation
Cool the required volume of acetone to -20°C.
Place protein sample in acetone-compatible tube.
Add four times the sample volume of cold (-20°C) acetone to the tube. Vortex tube and incubate for 60 minutes at -20°C.
Centrifuge 10 minutes at 13,000-15,000 x g.
Decant and properly dispose of the supernatant, being careful to not dislodge the protein pellet.
Allow the acetone to evaporate from the uncapped tube at room temperature for 30 minutes. Do
not over-dry pellet, or it may not dissolve properly.
Add buffer appropriate for the downstream process and vortex thoroughly to dissolve protein pellet.
The solution thus obtained is used as enzyme source for carrying out enzyme assay.
Enzyme Assay
To 1ml of enzyme source add 1ml of 1% casein solution along with 0.5 ml Tris-HCl Buffer.
Incubate the above mixture at 45°C for 30min.
Now add 4ml of 5% TCA (Trichloroacetic acid) and incubate at 30°C for 60min to stop the enzyme substrate reaction.
After incubation time filter the solution to yield TCA dissolved portion of assay mixture.
Take 1ml of this solution and add 5ml of 0.4M Na2CO3 and 0.5 ml of Phenol-Water (1:1) solution.
Now measure the OD of the resultant solution at 660nm immediately.
Enzyme Activity Calculation
For Bacillus subtilis the following studies were carried out
Effect of Purity of Enzyme on Protease activity
The Activity of crude enzyme, enzyme obtained from partial purification of Ammonium sulphate and Acetone were measured.
Effect of pH on Enzyme Activity
The activity of crude enzyme was measured at different pH values. The pH was adjusted using 0.1N HCl and 0.1N NaOH solutions and then checked for activity.
Effect of Temperature on Enzyme Activity and Stability
The activity of crude enzyme was determined by incubating the reaction mixture at different temperatures varying from 30°C to 70°C for 30min.
Effect of Moisture content on Enzyme Activity
The activity of crude enzyme from SSF was measured for different moisture contents in the
substrates ranging from 1:1, 1:1.5, 1:2, 1:2.5, 1:3 (Substrate: Moisture).
Effect of Inoculum size on Enzyme Activity
The activity of crude enzyme was determined at varying sizes of inoculum ranging from 10% to 40%.
Effect of Incubation Time on Enzyme Activity
The Activity was monitored at different time of incubation of organism ranging from 24 – 72 hrs in Bacillus subtilis and 12- 168 hrs for Aspergillus flavus.
Effect of Carbon source on Enzyme Activity
The change in the activity of crude enzyme grown under varying carbon sources (Starch, Maltose, Sucrose, Glucose) was studied.
Effect of Nitrogen source on Enzyme Activity
The change in the activity of crude enzyme grown under varying Nitrogen sources (Casein,
Thiourea, Peptone, Yeast Extract) was studied.
Effect of Surfactants on Enzyme Activity
The activity of crude enzyme was monitored in the presence of various surfactants like SDS, TritonX and Tween 80.
Effect of mutagenesis on Enzyme Activity
Bacillus subtilis was mutated using potent mutants Ethidium Bromide(5mg/ml) in concentrations 10μl/ml of inoculum and 20μl/ml of inoculum, exposed to UV light for 10 min and 20min respectively and also using Acrylamide(5mg/ml) in concentrations 10μl/ml of inoculum and 20μl/ml of inoculum and checked for enzyme activity.
Results and Discussion
Bacillus Subtilis
The standard graph for the Protease enzyme estimation was drawn as follows
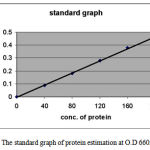 |
Figure 0: The standard graph of protein estimation at O.D 660nm.
|
From the above graph, the concentrations of the protease enzyme corresponding to the O.D at
0nm is determined and thus the activity of the enzyme estimated at different instances
Effect of incubation time on Enzyme Activity
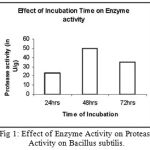 |
Figure 1: Effect of Enzyme Activity on Protease Activity on Bacillus subtilis.
|
The bacterium was grown for different time periods and then the assay was carried out. By carrying
out the enzyme assay at 660nm and corresponding activity was calculated. After the completion of
48hrs the highest enzyme activity was observed.
Effect of Temperature on Enzyme Activity
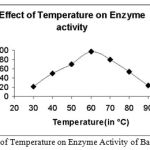 |
Figure 2: Effect of Temperature on Enzyme Activity of Bacillus subtilis.
|
The enzyme was subjected to different temperature conditions. The temperature was varied from
30-90°C and it was found that at 60°C the enzyme showed maximum activity.
Effect of Increased Temperature on Enzyme Activity
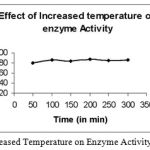 |
Figure 3: Effect of increased Temperature on Enzyme Activity of Bacillus subtilis.
|
The temperature was increased to 80°C and the incubation time was varied from 50 to 300 min and found that the activity remained almost constant.
Effect of pH on Enzyme Activity
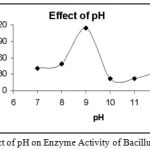 |
Figure 4: Effect of pH on Enzyme Activity of Bacillus subtilis.
|
The enzyme was subjected to different pH conditions. The pH was varied from 7-12 and it was found that at pH 9 the enzyme showed maximum activity.
Effect of Moisture content on Enzyme Activity
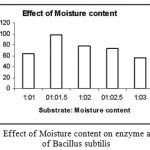 |
Figure 5: Effect of Moisture content on enzyme activity of Bacillus subtilis.
|
The enzyme was subjected to different substrate:moisture conditions. The moisture levels were varied from 1:1, 1:1.5, 1:2, 1:2.5, 1;3 and it was found that at Substrate:Moisture level of 1:1.5 the enzyme showed maximum activity.
Effect of Inoculum size on Enzyme Activity
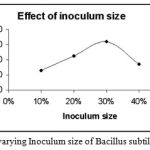 |
Figure 6: The Effect of varying Inoculum size of Bacillus subtilis on enzyme activity.
|
The inoculum size of the strain Bacillus subtilis was varied from 10% – 40%. The inoculum size that was found to be favourable for the maximum enzyme activity was 30%.
Effect of Purity on Enzyme Activity
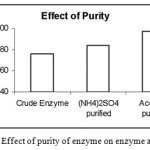 |
Figure 7: The Effect of purity of enzyme on enzyme activity.
|
The crude enzyme was subjected to partial purification techniques like precipitations by Ammonium Sulphate method and also Acetone method. Then the activity of crude enzyme, enzyme purified by Ammonium Sulphate precipitation and enzyme purified by Acetone precipitation was determined. It was found that the enzyme from Acetone precipitation is more pure followed by the enzyme purified by Ammonium Sulphate and then followed by crude enzyme.
Effect of various ‘C’ on Enzyme Activity
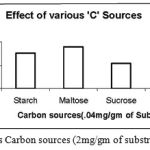 |
Figure 8: Effect of various Carbon sources (2mg/gm of substrate) on enzyme activity.
|
The Carbon sources were mixed with the wheat bran finely before moistening it. The different Carbon sources were added in the same concentration of 2mg/ gm of solid substrate. Out of the four Carbon sources Starch, Maltose, Sucrose and Glucose used, the one favourable for cultivation of Bacillus subtilis and also protease production was Maltose.
The carbon sources being rich in energy are easily consumed by the bacterial strains through several mechanisms are metabolized in their bodies.
Effect of various ‘N’ on Enzyme Activity
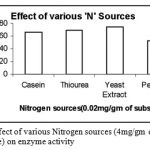 |
Figure 9: Effect of various Nitrogen sources (4mg/gm of substrate) on enzyme activity.
|
The Nitrogen sources were mixed with the wheat bran finely before moistening it. The different Nitrogen sources were added in the same concentration of 4mg/ gm of solid substrate. Out of the four Nitrogen sources Casein, Thiourea, Yeast Extract and Peptone used, the one favourable for cultivation of Bacillus subtilis and also protease production was Yeast Extract.
Effect of Surfactants on Enzyme Activity
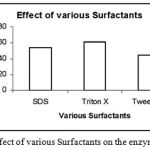 |
Figure 10: Effect of various Surfactants on the enzyme activity.
|
The enzyme was subjected to effects of various surfactants like SDS, Triton X and Tween 80. Out of these presence of Triton X enhanced the Enzyme activity to maximum.
Effect of Substrate concentration on Enzyme Activity
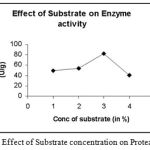 |
Figure 11: The Effect of Substrate concentration on Protease activity.
|
The substrate casein is specific substrate to protease. Thus it is used during the enzyme assay. It was added in varying concentrations during the assay of protease enzyme in concentrations varying from 1% – 4%. Out of these concentrations, 3% of casein concentration showed maximum activity.
Effect of Mutagenesis on Enzyme Activity
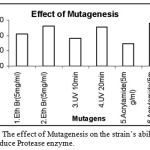 |
Figure 12: The effect of Mutagenesis on the strain’s ability to produce Protease enzyme.
|
The mutations play a major role in enhancing the enzyme activity. Various mutagens with varying concentrations were used for testing of the activity. The mutagens used were Exposure of strain to 10μl, 20μl of Ethidium Bromide of 5mg/ml concentration, exposure to UV light for 10 min and 20 min, Exposure to 10μl, 20μl of Acrylamide of 5mg/ml concentration. Out of these mutagens, the enzyme activity enhanced more with the Acrylamide at 20μl level followed by Ethidium Bromide at 20μl level and UV exposure for 20min.
References
- Adinarayana K, Ellaiah P. Production of alkaline protease by immobilized cells of alkalophilic Bacillus sp, J Sci Ind Res (India).
- Lowry OH, Rosebrough NJ, Farr AL, Randall RL. Protein measurement with the folin phenol reagent. J Biol Chem. 1951; 193:265-273.
- Debette J. Isolation and characterization of an extracellular protease produced by a soil strain. Curr Microbiol. 1991; 22:85-90.
- Levisohn, Sharon. Aronson, Arthur I. Regulation of Extracellular Protease Production in Bacillus substilis. J Bacteriol. 1967 Mar; 93(3):1023–1030.
- LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov; 193(1):265–275.
- MANDELL JD, GREENBERG J. A new chemical mutagen for bacteria, Ethidium bromide. Biochem Biophys Res Commun. 1960 Dec;3:575–577
- Morihara K. The specificities of various neutral and alkaline proteinases from microorganisms. Biochem Biophys Res Commun. 1967 Mar 21;26 (6):656–661.
- Young DB, Broadbent DA. Biochemical characterization of extracellular proteases from Bacillus substilis. Infect Immun. 1982 Sep;37 (3):875–883.
- Rinderknecht H, Geokas MC, Silverman P, Haverback BJ. A new ultrasensitive method for the determination of proteolytic activity. Clin Chim Acta. 1968 Aug;21(2):197–203.
- Cohan, F. M. (2002). What are bacterial species? Annu Rev Microbiol 56, 457–487.[CrossRef][Medline]
- Soares, Valeria F.; Castilho, Leda R.; Bon, Elba P. S.; Freire, Denise M. G. High-Yield Bacillus subtilis Protease Production by Solid-State Fermentation Applied Biochemistry and Biotechnology, Volume 121, Numbers 1-3, April 2005 , pp. 311-320(10).
- Y. Ellouz, A. Bayoudh, S. Kammoun, N. Gharsallah and M. Nasri. Production of protease by Bacillus subtilis grown on sardinelle heads and viscera flour Unité de Technologie Enzymatique et de Microbiologie, Ecole Nationale d’Ingénieurs de Sfax, B.P. “W”, 3038 Sfax, Tunisia.
- Maria Delia Pastor, Graciela Susana Lorda; Antonio Balatti. PROTEASE OBTENTION USING BACILLUS SUBTILIS 3411 AND AMARANTH SEED MEAL MEDIUM AT DIFFERENT AERATION RATES Braz. J. Microbiol. Vol.32 no 1 Sao Paulo Jan./Mar. 2001
- RODBELL, M., AND JONES, A. B., J. Biol. Chem., 241,140 (1966).
- JUNGAS, R. L., AND BALL, E. G., Biochemistry, 2, 383 (1963).
- Prakasham RS. Subba Rao Ch, Sreenivas Rao, R, Rajesham S, Sarma PN. Optimization of alkaline protease production by Bacillus sp using Taguchi methodology. Appl Biochem Biotechnol. 2005; 120:133-144.
- Ellaiah P, Srinivasulu B, Adinarayana K. A review on microbial alkaline proteases. J Sci Ind Res (India). 2002; 61:690-704.
- Ellaiah P, Adinarayana K, Pardhasaradhi SV, Srinivasulu B. Isolation of alkaline protease producing bacteria from Visakhapatnam soil. Ind J Microbiol. 2002; 42:173-175.
- Adinarayana K, Ellaiah P. Investigations on alkaline protease production with B subtilis PE-11 immobilised in calcium alginate gel beads. Process Biochem. 2004; 39:1331-1339.
- Sen S, Satyanarayana T. Optimization of alkaline protease production by thermophilic Bacillus licheniformis S-40. Indian J Microbiol. 1993;33:43-47.
- Tsuchida O, Yamagota Y, Ishizuka J, et al. An alkaline proteinase of an alkalophilic Bacillus sp. Curr Microbiol. 1986;14:7-12.
- Vuillemard JC, Terre S, Benoit S, Amiot J. Protease production by immobilized growing cells of Serratia marcescens and Myxococcus xanthus in calcium alginate gel beads. Appl Microbiol Biotechnol. 1988;27:423-431.
- Biochim Biophys Acta, 1980 Nov 7, 620(2), 332 – 7. Cardiolipin, a major phospholipid of Gram-positive bacteria that is not readily extractable; Filgueiras MH et al.
- Bacteriol, 1980 Oct, 144(1), 238 – 46. Effects of carbon source and growth rate on cell wall composition of Bacillus subtilis subsp. niger; Kruyssen FJ et al.