Manuscript accepted on :October 12, 2008
Published online on: 09-11-2015
Plagiarism Check: Yes
C .S. Felice*, Purbaj Pant, Bhim Gurung, Akshaya Neupane and Bijay Dhungel
Department of Biotechnology, Koneru lakshmaiah College of Engineering Vaddeswaram- 522502, Guntur Dist, Andhra Pradesh (India).
Corresponding Author E- mail: Shechinahfelice@yahoo.co.inAbstract
Urea has become the most used nitrogen fertilizer in the world, accounting for approximately 40% of the totally nitrogen supply. Its market share is increasing since it is the least expensive form of solid nitrogen fertilizer and its high nutrient content (46%N). Much of the nitrogen in fertilizer comes from urea, which bacteria degrade into ammonia and CO2 using urease.Its efficiency is however decreased by losses of nitrogen through ammonia volatilization by urease enzyme catalyzing it. In 1926 James Sumner showed that urease is a protein. Urease is found in bacteria, yeast and several higher plants. Urease is significant in the history of Enzymology as the first enzyme to be purified and crystallized. The present study was undertaken to study the isolation of the enzyme and the effect of various activators and inhibitors on the activity of the enzyme
Keywords
Urea; urease; Estimation; Modulators; Inhibition; spectrophotometry
Download this article as:| Copy the following to cite this article: Felice C. S, Pant P, Gurung B, Neupane A, Dhungel B.Effect of Organic Additives on Activity of Urease.Biomed. Pharmacol. J.;1(2) |
| Copy the following to cite this URL: Felice C. S, Pant P, Gurung B, Neupane A, Dhungel B.Effect of Organic Additives on Activity of Urease.Biomed. Pharmacol. J.;1(2) Available from: http://biomedpharmajournal.org/?p=514 |
Introduction
In 1926 James Sumner showed that urease is a protein. Urease is found in bacteria, yeast and several higher plants.urease is significant in the history of enzymology as the first enzyme to be purified and crystallized. James B. Sumner of Cornell University in (1946) received the Nobel Prize for his work with the enzyme urease, extracted from the jack bean. Urease is an enzyme that catalyzes the conversion of urea to ammonia and carbon dioxide. Certain bacteria that convert urea to ammonia as part of the nitrogen cycle contain this enzyme.Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB) has given nomenclature to the urease as E.C (3.5.1.5).Enzyme commission 3.5 of enzyme urease denotes its action that it is a hydrolase which breaks the bond by addition of water molecule(no:3) and specifically breaks carbon-nitrogen non peptide bond.(no:5). An unusual feature of urease is its dependence on nickel to grab onto and break up urea in the enzyme’s active site. In 1982, Australian researchers Barry Marshall and Robin Warren discovered spiral- shaped bacteria in the stomach, later named Helicobacter pylori (H. pylori). After closely studying H. pylori’s effect on the stomach, they proposed that the bacteria were the underlying cause of gastritis and peptic ulcers by using enzyme urease.
Materials and Methods
Beans of Dolichos lab lab, Nesslers reagent,. Tris acetate buffer (10%TCA), 4. Sulphuric Acid (1N H2SO4), phosphate buffer (0.2M), ammonium sulphate solution, pure enzyme urease (0.1%)
Experimental
Urease enzyme was isolated from seeds of beans using mortar pestle and phosphate buffer and then stored at 4 C .Standardization of ammonium sulphate using Nessler’s reagent will be done by drawing standard graph.Then determination of urease activity under various modulators with substrate (urea) by using the standard curve. Then determination of pure urease activity under the same modulators. Then comparison of the results obtained.
Determination of urease activity
3 clean test tubes are taken and marked as control, test, blank. To the tubes control and test 2 ml of urea substrate were added for the blank 4.5 ml of phosphate buffer was added. The tubes were incubated for 10 min and add 0.5ml of enzyme to the test.. After 15 min 0.5 ml of enzyme was added to the tube control and immediately to all the tubes 0.5 ml of 10% trichloro acetic acid and 0.5 ml of 1N H2SO4 were added. All the tubes were centrifuged for 10 min at 2000rpm. After centrifugation 0.5 ml of supernatant was taken in a tube separately from all the test tubes. To the supernatant 0.5 ml of Nesslers reagent was added and the yellow colour developed was measured at 540 nm.
Results and Discussion
After performing the said experiments, to the enzyme sample-varying conceentration of the inhibitor glycerol was taken and the activity of the enzyme was studied under specified and defined conditions. Enzyme activity is measured at various time intervals like 15min, 30min, 45min and maximum enzyme activity is shown at 30min, decrease for 45minutes. The results of analysis are presented in Table -1
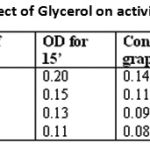 |
Table 1: Effect of Glycerol on activity of urease.
|
Conclusion
Temperature fluctuations have significant effect on enzyme activity. Following conclusions are drawn for the enzyme activity and effect of inhibitors on enzyme activity for crude enzyme source and pure enzyme. Glycerol showed maximum inhibition at 20% concentration for crude enzyme source, and for pure enzyme maximum inhibition is shown at 20%.
Acknowledgements
The authors are grateful to Management of Koneru Lakshmaiah College of Engineering, Vaddeswaram, Guntur Dist, for their continuous support and encouragement and for providing the necessary facilities for carrying out the project.
References
- BURNE, R.A. AND CHEN, Y.Y. Bacterial ureases in infectious diseases. MICROBES INFECT. 2 533-542 (2000).
- MOBLEY, H.L., ISLAND, M.D. AND HAUSINGER, R.P. Molecular biology of microbial, ureases. MICROBIOL.REV. 59 451-480 (1995).
- MOBLEY, H.L., ISLAND, M.D. AND MASSAD, G. Virulence determinants of uropathogenic, Escherichia coli and Proteus mirabilis. KIDNEY INT.SUPPL. 47 S129-136 (1994).
- MCGEE, D.J. AND MOBLEY, H.L. Mechanisms of Helicobacter pylori infection: bacterial, factors. R.TOP.MICROBIOL.IMMUNOL. 241 155-180 (1999).
- HA, N.C., OH, S.T., SUNG, J.Y., CHA, K.A., LEE, M.H. AND OH, B.H. Supramolecular assembly and acid resistance of Helicobacter pylori urease. NAT.STRUCT.BIOL. 8 505-509 , (2001) .
- P Bauerfeind, R Garner, B E Dunn, and H L Mobley, Synthesis and activity of Helicobacter pylori urease and catalase at low pH Gut. 1997 January; 40(1):25–30.
- Susanne Klose, M.A. Tabatabai, Soil Biology and Biochemistry, Volume , 31, issue 2, February 99, Pages 205-211.
- A. Rivadeneyra, A. Gutierrez-Calderón, A.M. Rivadeneyra, A. Ramos-Cormenzana, A Study of Struvite Precipitation and Urease Activity in Bacteria Isolated from Patients with Urinary Infections and Their Possible Involvement in the Formation of Renal Calculi, Urologia Internationalis, Vol-63, No.3 1999.
- K. Sanyal, B. Banerjee, Urease activity in Trichophyton Mentagrophytes, folia microbiologica, Volume 28, number 2 , march 1983.







