Manuscript accepted on :January 21, 2009
Published online on: --
Plagiarism Check: Yes
Nadeem Siddiqui¹*, Arpana Rana¹, Suroor A. Khan¹, Waquar Ahsan¹, M. Shamsher Alam¹ and Sharique Ahmed2
1Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Jamia Hamdard (Hamdard University), Hamdard Nagar, New Delhi
2Department of Biochemistry, Faculty of Medicine, 7th October University, Misurata, Libya (India).
Corresponding Author E-mail:nadeems_03@rediffmail.com
Abstract
Various benzothiazole-benzamides synthesized were evaluated for their analgesic and antidepressant activities. Interestingly, all the compounds showed promising activities against the two screenings. Some displayed highly significant activities when compared to the standard.
Keywords
Analgesic; antidepressant activities of benzothiazole-benzamides
Download this article as:| Copy the following to cite this article: Siddiqui N, Rana A, Khan S. A, Ahsan W, Alam M. S, Ahmed S. Analgesic and Antidepressant Activities of Benzothiazole-Benzamides. Biomed. Pharmacol. J.2008;1(2) |
| Copy the following to cite this URL: Siddiqui N, Rana A, Khan S. A, Ahsan W, Alam M. S, Ahmed S. Analgesic and Antidepressant Activities of Benzothiazole-Benzamides. Biomed. Pharmacol. J.2008;1(2). Available from: http://biomedpharmajournal.org/?p=429 |
Introduction
Benzothiazole derivatives in recent years have acquired conspicuous significance due to their wide spectrum of biological activities including anticonvulsant, analgesic, and antidepressant activities.1,2 Benzamides on the other hand had also been found to show a wide range of pharmacological activities such as anticonvulsant3, anti-inflammatory, 4 analgesic, 5 and antidepressant activity. 6
The present series was designed previously by combining the two pharmacologically active pharmacophores to get benzothiazole-benzamide derivatives and their anticonvulsant activity of them has been reported.7
Since the results were found very encouraging, we intended to test these compounds for other pharmacological activities. This paper presents the analgesic and antidepressant activities of the synthesized benzothiazole-benzamides (Fig. 1).
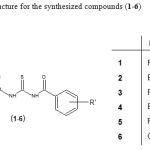 |
Figure 1: General structure for the synthesized compounds (1-6).
|
Materials and Methods
Analgesic activity
The analgesic activity was assessed by using Thermal stimulus technique.8 The investigations were done on albino mice in groups of 6 each which were kept under standard laboratory conditions. The test compounds were suspended in methyl cellulose-water (0.5 %) mixture. Each compound was administered orally at a dose of 20 mg/kg. The analgesic activity was assessed after 4 h interval of the administration. Tail of each mice was gently immersed into thermostatically controlled water at 55 °C. The parameter measured in test samples was time that elapsed between immersion and the attempt to withdraw the tail from hot water for control as well as treated group of animals.
Antidepressant activity
The antidepressant activity was studied on albino rats of either sex (110-140 g) in group of 6 each by forced swimming test/despair swim test.9 The forced swimming test consisted of placing rats, individually in plexiglass cylinders (40 cm high, 20 cm in diameter) containing water (24-26 °C, 30 cm deep) for two swimming sessions: an initial 15 min training session, which was followed 24 h later, by a 5 min test session after 1 h of drug administration, the animals were removed from the cylinder dried with paper towels, placed in an individual cage to rest and recover for 15 min and was noted. Immobility time is the time spent by rat floating in water without struggling, making those movements necessary to keep the head above the water.
Results and Discussion
Computational parameters
Various computational parameters were calculated by using software ACD labs version 8.0 and the values are presented in Table 1. All the values were found to be in acceptable range. Fig 2 depicts the 3D optimized general structure of the synthesized compounds.
 |
Figure 2: Three dimensional optimized general structure of the synthesized N-(6-substituted-1,3-benzothiazol-2-ylcarbamothioyl)-2/4-substituted benzamides (1-6)
|
Table 1: Computational data of compounds (1-6)
| Compound | aMolar Refractivity (cm3) | bMolar Volume (cm3) | cIndex of Refraction | dSurface Tension (dyne/cm) | eDensity (g/cm3) | fPolarizability (cm3) |
| 1 | 90.65 | 219.6 | 1.763 | 76.4 | 1.508 | 35.93 |
| 2 | 100.23 | 243.5 | 1.790 | 80.1 | 1.752 | 40.93 |
| 3 | 95.55 | 231.5 | 1.762 | 76.6 | 1.579 | 37.87 |
| 4 | 100.24 | 243.5 | 1.790 | 80.1 | 1.752 | 40.93 |
| 5 | 95.55 | 231.5 | 1.762 | 76.6 | 1.579 | 37.83 |
| 6 | 104.02 | 263.4 | 1.719 | 67.7 | 1.417 | 41.23 |
All properties are calculated from ACD labs version 8.0.aMolar refractivity within ± 0.3 cm3 range; bMolar volume within ± 0.3 cm3 range; cIndex of refraction within ± 0.02 range; dSurface tension within ± 0.3 dyne/cm range; eDensity within ± 0.06g/ cm3 range; fPolarizabilty within ± 0.5 × 10-24 cm3 range.
Analgesic activity
All the compounds showed promising analgesic activity when tested and compared with the standard drug diclofenac and the results are presented in Table 2. Compounds 3 and 4 were found to be highly potent with p < 0.001, whereas, compounds 1, 2, 5 and 6 showed significant analgesic activity with p < 0.01.
Table 2: Analgesic and antidepressant activities of compounds (1-6).
| Compound | Analgesic activitya | Antidepressant activityb | ||
| Mean ± SEMc | Mean ± SEMd | |||
| Control | Treated | Control | Treated | |
| 1 | 11 ± 1.40 | 19.0 ± 1.840** | 34.4 ± 1.490 | 24.0 ± 1.640 |
| 2 | 2.9 ± 0.341 | 4.4 ± 0.461** | 42.0 ± 1.267 | 22.6 ± 1.002*** |
| 3 | 3.6 ± 0.477 | 7.7 ± 0.470*** | 34.6 ± 1.670 | 25.0 ± 1.433* |
| 4 | 2.2 ± 0.177 | 5.4 ± 0.481*** | 48.2 ± 2.504 | 38.0 ± 2.378** |
| 5 | 2.7 ± 0.524 | 6.1 ± 0.663** | 39.8 ± 1.332 | 24.6 ± 1.484** |
| 6 | 2.7 ± 0.521 | 6.8 ± 0.660** | 23.2 ± 0.827 | 16.8 ± 0.942*** |
| Diclofenac | 1.8 ± 0.20 | 19.6 ± 0.890*** | — | — |
| Fluoxetine | — | — | 193.6 ± 0.640 | 24.2 ± 4.99*** |
n = 6; aDose = 20 mg/kg (p.o.); bDose = 30 mg/kg (p.o.) cMean average reaction time (sec.); dMean average immobility time (sec). *p < 0.05, **p < 0.01, ***p < 0.001. Data was analyzed by student’s unpaired ‘t’ test.
Antidepressant activity
Antidepressant activity of all the titled compounds was evaluated and the results are shown in Table 2. Compounds 2 and 6 showed significant decrease in the immobility time and were found to be highly potent (p < 0.001) comparable with the standard drug fluoxetine. Compounds 4 and 5 were also found to be having significant antidepressant activity with p < 0.01. Results of the rest of the compounds were not significant.
Conclusion
A newer series of compounds were synthesized and evaluated as analgesics and antidepressant agents. Some compounds have shown encouraging activities and are needed to be investigated further to get better agents that can have strong future commitments.
Acknowledgments
Financial assistance provided by University Grants Commission (UGC) is gratefully acknowledged.
Referencess
- Sawhney, S. N., Bhutani, S. and Dharamvir. “Synthesis of some 2-(2-benzothiazolyl) and 2-(2-benzimidazolyl)-6-aryl-4,5-dihydro-3(2H)-pyridazinones as potential anti-inflammatory agents.” J. Chem. 26B, 348-350 (1987).
- Singh, S. P. and Vaid, R. K. “Synthesis and anti-inflammatory activity of some 2-(4’-butyl-3’,5’-dimethylpyrazol-1’yl)-6-substituted benzothiazoles and 4-butyl-1-(6’-substituted-2’-benzothiazolyl)-3-methylpyrazol-5-ones.” J. Chem. 25B, 288-291 (1986).
- Foster, J. E., Nicholson, J. M., Butcher, R., Stables, J. P. and Edafiogho, I. O. “Synthesis, characterization and anticonvulsant activity of enaminones part 6: Synthesis of substituted vinylic benzamides as potential anticonvulsants.” Med. Chem. 7, 2415-2425 (1999).
- Caliendo, G., Santagada, V., Perissuti, E., Severino, B., Fiorino, F. and Warner, T. D. “Synthesis of substituted benzamides as anti-inflammatory agents that inhibit preferentially cyclooxygenase-I but do not cause gastric damage.” J. Med. Chem. 36, 517-530 (2001).
- Coats, S. J., Schulz, M. J., Carson, J. R., Codd, E. E. Hlasta, D. J. and Pitis, P. M. “Dax, Parellel methods for the preparation and SAR exploration of N-ethyl-4-[(8-alkyl)-8-aza-bicyclo[3.2.1]-oct-3-ylidene) aryl-methyl]-benzamides, powerful µ and Δ opioid agonists.” Med. Chem. Lett. 14, 2109-2112 (2004).
- Sonda, S., Kawahara, T., Katayama, K., Sato, N. and Asano, K. “Synthesis and pharmacological evaluation of benzamide derivatives as selective 5-HT4 receptor agonists.” Med. Chem. 13, 3295-3308 (2005).
- Rana, A., Siddiqui, N., Khan, S. A., Haque, S. E. and Bhat, M. A. “N-{[(6-Substituted-1,3-benzothiazole-2-yl)amino]carbonothioyl}-2/4-substituted benzamides: synthesis and pharmacological evaluation.” J. Med. Chem. 43, 1114-1122 (2008).
- Kendall, D. A., Browner, M. and Enna, S. J. “Comparison of antinociceptive effect og gamma-aminobutyric acid (GABA) agonists: evidence for a cholinergic involvement.” Pharmacol. Exp. Ther. 220, 482-487 (1982).
- Porsolt, R. D., Le Pichon, M., and Jalfre, M. “A new animal model sensitive to antidepressant treatments.” Nature 266, 730-732 (1977).
- Winter, C. A., Risley, E. A. and Nus, G. N. “Carrageenan induced edema in hind paw of the rat as an assay for anti-inflammatory drugs.” Soc. Exp. Biol. 111, 544-546 (1962).







